Page 332 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 332
Nanomaterials for Groundwater Remediation 317
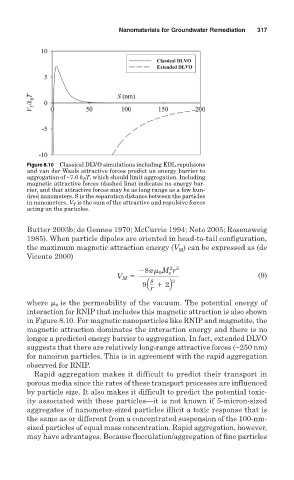
Figure 8.10 Classical DLVO simulations including EDL repulsions
and van der Waals attractive forces predict an energy barrier to
aggregation of ~7.0 k B T, which should limit aggregation. Including
magnetic attractive forces (dashed line) indicates no energy bar-
rier, and that attractive forces may be as long range as a few hun-
dred nanometers. S is the separation distance between the particles
in nanometers. V T is the sum of the attractive and repulsive forces
acting on the particles.
Butter 2003b; de Gennes 1970; McCurrie 1994; Neto 2005; Rosensweig
1985). When particle dipoles are oriented in head-to-tail configuration,
the maximum magnetic attraction energy (V ) can be expressed as (de
M
Vicente 2000)
2 3
28pm 0 M s r
5 (9)
V M
s 3
9Q 1 2R
r
where m is the permeability of the vacuum. The potential energy of
0
interaction for RNIP that includes this magnetic attraction is also shown
in Figure 8.10. For magnetic nanoparticles like RNIP and magnetite, the
magnetic attraction dominates the interaction energy and there is no
longer a predicted energy barrier to aggregation. In fact, extended DLVO
suggests that there are relatively long-range attractive forces (~250 nm)
for nanoiron particles. This is in agreement with the rapid aggregation
observed for RNIP.
Rapid aggregation makes it difficult to predict their transport in
porous media since the rates of these transport processes are influenced
by particle size. It also makes it difficult to predict the potential toxic-
ity associated with these particles—it is not known if 5-micron-sized
aggregates of nanometer-sized particles illicit a toxic response that is
the same as or different from a concentrated suspension of the 100-nm-
sized particles of equal mass concentration. Rapid aggregation, however,
may have advantages. Because flocculation/aggregation of fine particles