Page 336 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 336
Nanomaterials for Groundwater Remediation 321
Figure 8.12 Surface modification by strong polyelectrolyte polymers
enhances transport of nanoiron (RNIP) transport through saturated
porous media. Polymer-modified iron (striped bars) is highly trans-
portable through water-saturated sand columns, while unmodified
nanoiron (black bars) does not transport well through the column, par-
ticularly at high concentrations (3 g/L).
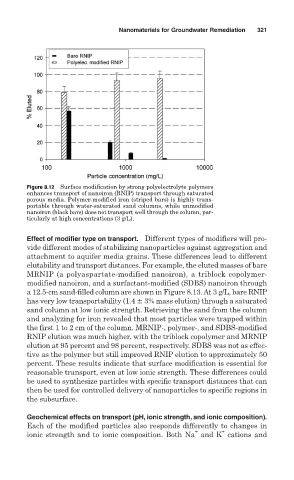
Effect of modifier type on transport. Different types of modifiers will pro-
vide different modes of stabilizing nanoparticles against aggregation and
attachment to aquifer media grains. These differences lead to different
elutability and transport distances. For example, the eluted masses of bare
MRNIP (a polyaspartate-modified nanoiron), a triblock copolymer-
modified nanoiron, and a surfactant-modified (SDBS) nanoiron through
a 12.5-cm sand-filled column are shown in Figure 8.13. At 3 g/L, bare RNIP
has very low transportability (1.4 ! 3% mass elution) through a saturated
sand column at low ionic strength. Retrieving the sand from the column
and analyzing for iron revealed that most particles were trapped within
the first 1 to 2 cm of the column. MRNIP-, polymer-, and SDBS-modified
RNIP elution was much higher, with the triblock copolymer and MRNIP
elution at 95 percent and 98 percent, respectively. SDBS was not as effec-
tive as the polymer but still improved RNIP elution to approximately 50
percent. These results indicate that surface modification is essential for
reasonable transport, even at low ionic strength. These differences could
be used to synthesize particles with specific transport distances that can
then be used for controlled delivery of nanoparticles to specific regions in
the subsurface.
Geochemical effects on transport (pH, ionic strength, and ionic composition).
Each of the modified particles also responds differently to changes in
+
+
ionic strength and to ionic composition. Both Na and K cations and