Page 341 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 341
326 Environmental Applications of Nanomaterials
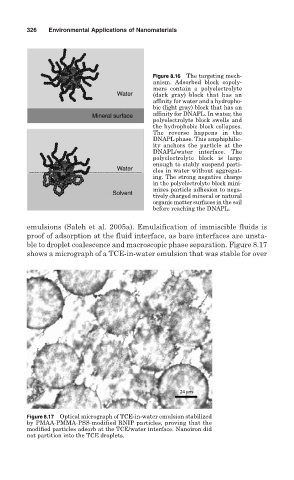
Figure 8.16 The targeting mech-
anism. Adsorbed block copoly-
mers contain a polyelectrolyte
(dark gray) block that has an
affinity for water and a hydropho-
bic (light gray) block that has an
affinity for DNAPL. In water, the
polyelectrolyte block swells and
the hydrophobic block collapses.
The reverse happens in the
DNAPL phase. This amphiphilic-
ity anchors the particle at the
DNAPL/water interface. The
polyelectrolyte block is large
enough to stably suspend parti-
cles in water without aggregat-
ing. The strong negative charge
in the polyelectrolyte block mini-
mizes particle adhesion to nega-
tively charged mineral or natural
organic matter surfaces in the soil
before reaching the DNAPL.
emulsions (Saleh et al. 2005a). Emulsification of immiscible fluids is
proof of adsorption at the fluid interface, as bare interfaces are unsta-
ble to droplet coalescence and macroscopic phase separation. Figure 8.17
shows a micrograph of a TCE-in-water emulsion that was stable for over
Figure 8.17 Optical micrograph of TCE-in-water emulsion stabilized
by PMAA-PMMA-PSS-modified RNIP particles, proving that the
modified particles adsorb at the TCE/water interface. Nanoiron did
not partition into the TCE droplets.