Page 337 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 337
322 Environmental Applications of Nanomaterials
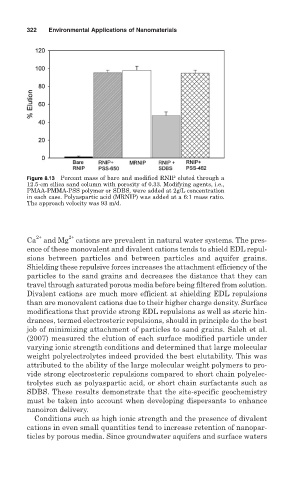
Figure 8.13 Percent mass of bare and modified RNIP eluted through a
12.5-cm silica sand column with porosity of 0.33. Modifying agents, i.e.,
PMAA-PMMA-PSS polymer or SDBS, were added at 2g/L concentration
in each case. Polyaspartic acid (MRNIP) was added at a 6:1 mass ratio.
The approach velocity was 93 m/d.
2+ 2+
Ca and Mg cations are prevalent in natural water systems. The pres-
ence of these monovalent and divalent cations tends to shield EDL repul-
sions between particles and between particles and aquifer grains.
Shielding these repulsive forces increases the attachment efficiency of the
particles to the sand grains and decreases the distance that they can
travel through saturated porous media before being filtered from solution.
Divalent cations are much more efficient at shielding EDL repulsions
than are monovalent cations due to their higher charge density. Surface
modifications that provide strong EDL repulsions as well as steric hin-
drances, termed electrosteric repulsions, should in principle do the best
job of minimizing attachment of particles to sand grains. Saleh et al.
(2007) measured the elution of each surface modified particle under
varying ionic strength conditions and determined that large molecular
weight polyelectrolytes indeed provided the best elutability. This was
attributed to the ability of the large molecular weight polymers to pro-
vide strong electrosteric repulsions compared to short chain polyelec-
trolytes such as polyaspartic acid, or short chain surfactants such as
SDBS. These results demonstrate that the site-specific geochemistry
must be taken into account when developing dispersants to enhance
nanoiron delivery.
Conditions such as high ionic strength and the presence of divalent
cations in even small quantities tend to increase retention of nanopar-
ticles by porous media. Since groundwater aquifers and surface waters