Page 369 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 369
354 Environmental Applications of Nanomaterials
term for RO and NF applications can be incorporated into an osmotic
pressure term in the numerator, and the following expression is
obtained:
s p 2 s "d
J 5 (34)
m5R m std 1 R c [d c std, c]6
Concentration polarization may be indirectly related to irreversible
fouling through its effects on adsorption, cake formation, and precipi-
tation. However, reductions in permeate flux (or increases in TMP) due
directly to CP are completely reversible, making this resistance term
quite different from the others.
Membranes in fuel cell applications
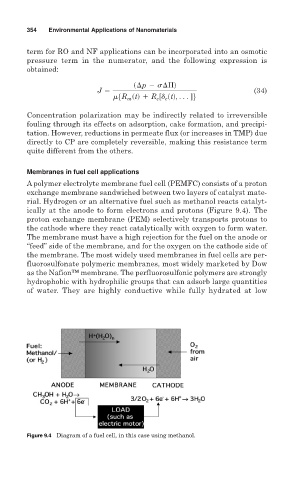
A polymer electrolyte membrane fuel cell (PEMFC) consists of a proton
exchange membrane sandwiched between two layers of catalyst mate-
rial. Hydrogen or an alternative fuel such as methanol reacts catalyt-
ically at the anode to form electrons and protons (Figure 9.4). The
proton exchange membrane (PEM) selectively transports protons to
the cathode where they react catalytically with oxygen to form water.
The membrane must have a high rejection for the fuel on the anode or
“feed” side of the membrane, and for the oxygen on the cathode side of
the membrane. The most widely used membranes in fuel cells are per-
fluorosulfonate polymeric membranes, most widely marketed by Dow
as the Nafion™ membrane. The perfluorosulfonic polymers are strongly
hydrophobic with hydrophilic groups that can adsorb large quantities
of water. They are highly conductive while fully hydrated at low
Figure 9.4 Diagram of a fuel cell, in this case using methanol.