Page 381 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 381
366 Environmental Applications of Nanomaterials
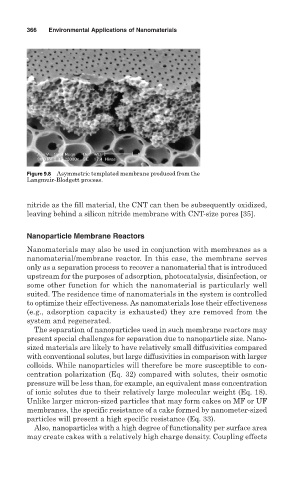
Figure 9.8 Asymmetric templated membrane produced from the
Langmuir-Blodgett process.
nitride as the fill material, the CNT can then be subsequently oxidized,
leaving behind a silicon nitride membrane with CNT-size pores [35].
Nanoparticle Membrane Reactors
Nanomaterials may also be used in conjunction with membranes as a
nanomaterial/membrane reactor. In this case, the membrane serves
only as a separation process to recover a nanomaterial that is introduced
upstream for the purposes of adsorption, photocatalysis, disinfection, or
some other function for which the nanomaterial is particularly well
suited. The residence time of nanomaterials in the system is controlled
to optimize their effectiveness. As nanomaterials lose their effectiveness
(e.g., adsorption capacity is exhausted) they are removed from the
system and regenerated.
The separation of nanoparticles used in such membrane reactors may
present special challenges for separation due to nanoparticle size. Nano-
sized materials are likely to have relatively small diffusivities compared
with conventional solutes, but large diffusivities in comparison with larger
colloids. While nanoparticles will therefore be more susceptible to con-
centration polarization (Eq. 32) compared with solutes, their osmotic
pressure will be less than, for example, an equivalent mass concentration
of ionic solutes due to their relatively large molecular weight (Eq. 18).
Unlike larger micron-sized particles that may form cakes on MF or UF
membranes, the specific resistance of a cake formed by nanometer-sized
particles will present a high specific resistance (Eq. 33).
Also, nanoparticles with a high degree of functionality per surface area
may create cakes with a relatively high charge density. Coupling effects