Page 46 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 46
32 Principles and Methods
In these equilibria, h is the hydroxylation ratio of the cation. It rep-
resents the number of hydroxo ligands (OH) present within the coordi-
nation sphere, or the number of protons eliminated from the coordination
sphere of the aqua cation.
The acidity of the aqua cation strongly depends on the strength of the
M-O bond—that is, the magnitude of the electron transfer from oxygen
toward the metal. The acidity can be related to the polarizing charac-
ter of the cation—that is, the ratio of formal charge (oxidation state) to
its size. The equilibrium constant of the first step of hydroxylation
(h 1) for many cations where d stands for the M-O distance can be
9
empirically expressed by:
log K ≈ 20 11(z/d) G RT
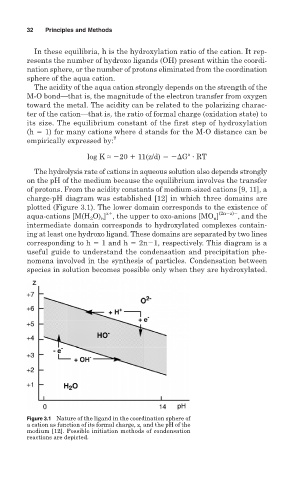
The hydrolysis rate of cations in aqueous solution also depends strongly
on the pH of the medium because the equilibrium involves the transfer
of protons. From the acidity constants of medium-sized cations [9, 11], a
charge-pH diagram was established [12] in which three domains are
plotted (Figure 3.1). The lower domain corresponds to the existence of
z
aqua-cations [M(H O) ] , the upper to oxo-anions [MO ] (2n z) ,and the
n
n
2
intermediate domain corresponds to hydroxylated complexes contain-
ing at least one hydroxo ligand. These domains are separated by two lines
corresponding to h 1 and h 2n 1, respectively. This diagram is a
useful guide to understand the condensation and precipitation phe-
nomena involved in the synthesis of particles. Condensation between
species in solution becomes possible only when they are hydroxylated.
Figure 3.1 Nature of the ligand in the coordination sphere of
a cation as function of its formal charge, z, and the pH of the
medium [12]. Possible initiation methods of condensation
reactions are depicted.