Page 48 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 48
34 Principles and Methods
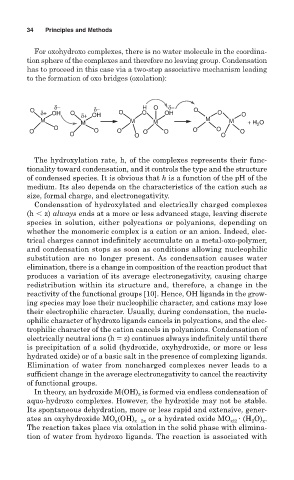
For oxohydroxo complexes, there is no water molecule in the coordina-
tion sphere of the complexes and therefore no leaving group. Condensation
has to proceed in this case via a two-step associative mechanism leading
to the formation of oxo bridges (oxolation):
δ− δ− H O δ−
O δ+ OH O δ+ OH O O OH O O O
M M M M M M + H 2 O
O O
O O O O O O O
O O O
The hydroxylation rate, h, of the complexes represents their func-
tionality toward condensation, and it controls the type and the structure
of condensed species. It is obvious that h is a function of the pH of the
medium. Its also depends on the characteristics of the cation such as
size, formal charge, and electronegativity.
Condensation of hydroxylated and electrically charged complexes
(h z) always ends at a more or less advanced stage, leaving discrete
species in solution, either polycations or polyanions, depending on
whether the monomeric complex is a cation or an anion. Indeed, elec-
trical charges cannot indefinitely accumulate on a metal-oxo-polymer,
and condensation stops as soon as conditions allowing nucleophilic
substitution are no longer present. As condensation causes water
elimination, there is a change in composition of the reaction product that
produces a variation of its average electronegativity, causing charge
redistribution within its structure and, therefore, a change in the
reactivity of the functional groups [10]. Hence, OH ligands in the grow-
ing species may lose their nucleophilic character, and cations may lose
their electrophilic character. Usually, during condensation, the nucle-
ophilic character of hydroxo ligands cancels in polycations, and the elec-
trophilic character of the cation cancels in polyanions. Condensation of
electrically neutral ions (h z) continues always indefinitely until there
is precipitation of a solid (hydroxide, oxyhydroxide, or more or less
hydrated oxide) or of a basic salt in the presence of complexing ligands.
Elimination of water from noncharged complexes never leads to a
sufficient change in the average electronegativity to cancel the reactivity
of functional groups.
In theory, an hydroxide M(OH) is formed via endless condensation of
z
aquo-hydroxo complexes. However, the hydroxide may not be stable.
Its spontaneous dehydration, more or less rapid and extensive, gener-
ates an oxyhydroxide MO (OH) z 2x or a hydrated oxide MO z/2 (H O) .
x
x
2
The reaction takes place via oxolation in the solid phase with elimina-
tion of water from hydroxo ligands. The reaction is associated with