Page 50 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 50
36 Principles and Methods
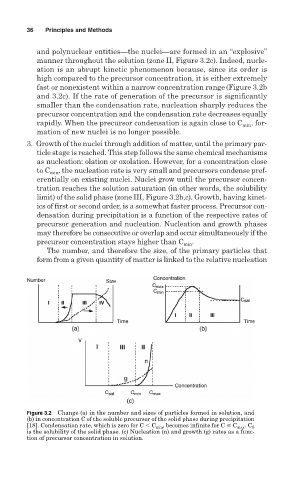
and polynuclear entities—the nuclei—are formed in an “explosive”
manner throughout the solution (zone II, Figure 3.2c). Indeed, nucle-
ation is an abrupt kinetic phenomenon because, since its order is
high compared to the precursor concentration, it is either extremely
fast or nonexistent within a narrow concentration range(Figure 3.2b
and 3.2c). If the rate of generation of the precursor is significantly
smaller than the condensation rate, nucleation sharply reduces the
precursor concentration and the condensation rate decreases equally
rapidly. When the precursor condensation is again close to C min , for-
mation of new nuclei is no longer possible.
3. Growth of the nuclei through addition of matter, until the primary par-
ticle stage is reached. This step follows the same chemical mechanisms
as nucleation: olation or oxolation. However, for a concentration close
to C min , the nucleation rate is very small and precursors condense pref-
erentially on existing nuclei. Nuclei grow until the precursor concen-
tration reaches the solution saturation (in other words, the solubility
limit) of the solid phase (zone III, Figure 3.2b,c). Growth, having kinet-
ics of first or second order, is a somewhat faster process. Precursor con-
densation during precipitation is a function of the respective rates of
precursor generation and nucleation. Nucleation and growth phases
may therefore be consecutive or overlap and occur simultaneously if the
precursor concentration stays higher than C min .
The number, and therefore the size, of the primary particles that
form from a given quantity of matter is linked to the relative nucleation
Figure 3.2 Change (a) in the number and sizes of particles formed in solution, and
(b) in concentration C of the soluble precursor of the solid phase during precipitation
[18]. Condensation rate, which is zero for C C min , becomes infinite for C
C max . C S
is the solubility of the solid phase. (c) Nucleation (n) and growth (g) rates as a func-
tion of precursor concentration in solution.