Page 55 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 55
Nanomaterials Fabrication 41
2
medium and anaerobic conditions [29], surface Fe ions are desorbed
as hexa-aquo complexes in solution (electron and ion transfer) accord-
ing to:
3 2.5 3 3
[Fe ] [Fe 2 ] O 2H → 0.75 [Fe ] [Fe 5/3 V ] O 4
Oh
4
1/3 Oh
Td
Td
2
Fe aq H O
2
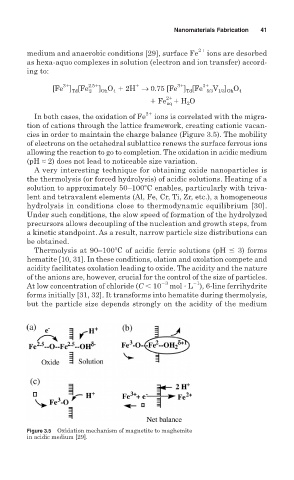
In both cases, the oxidation of Fe 2 ions is correlated with the migra-
tion of cations through the lattice framework, creating cationic vacan-
cies in order to maintain the charge balance (Figure 3.5). The mobility
of electrons on the octahedral sublattice renews the surface ferrous ions
allowing the reaction to go to completion. The oxidation in acidic medium
(pH ≈ 2) does not lead to noticeable size variation.
A very interesting technique for obtaining oxide nanoparticles is
the thermolysis (or forced hydrolysis) of acidic solutions. Heating of a
solution to approximately 50–100 C enables, particularly with triva-
lent and tetravalent elements (Al, Fe, Cr, Ti, Zr, etc.), a homogeneous
hydrolysis in conditions close to thermodynamic equilibrium [30].
Under such conditions, the slow speed of formation of the hydrolyzed
precursors allows decoupling of the nucleation and growth steps, from
a kinetic standpoint. As a result, narrow particle size distributions can
be obtained.
Thermolysis at 90–100 C of acidic ferric solutions (pH 3) forms
hematite [10, 31]. In these conditions, olation and oxolation compete and
acidity facilitates oxolation leading to oxide. The acidity and the nature
of the anions are, however, crucial for the control of the size of particles.
1
At low concentration of chloride (C 10 3 mol L ), 6-line ferrihydrite
forms initially [31, 32]. It transforms into hematite during thermolysis,
but the particle size depends strongly on the acidity of the medium
Figure 3.5 Oxidation mechanism of magnetite to maghemite
in acidic medium [29].