Page 57 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 57
Nanomaterials Fabrication 43
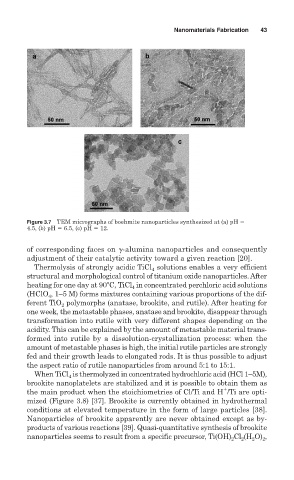
Figure 3.7 TEM micrographs of boehmite nanoparticles synthesized at (a) pH
4.5, (b) pH 6.5, (c) pH 12.
of corresponding faces on -alumina nanoparticles and consequently
adjustment of their catalytic activity toward a given reaction [20].
Thermolysis of strongly acidic TiCl solutions enables a very efficient
4
structural and morphological control of titanium oxide nanoparticles. After
heating for one day at 90 C, TiCl in concentrated perchloric acid solutions
4
, 1–5 M) forms mixtures containing various proportions of the dif-
(HClO 4
ferent TiO polymorphs (anatase, brookite, and rutile). After heating for
2
one week, the metastable phases, anatase and brookite, disappear through
transformation into rutile with very different shapes depending on the
acidity. This can be explained by the amount of metastable material trans-
formed into rutile by a dissolution-crystallization process: when the
amount of metastable phases is high, the initial rutile particles are strongly
fed and their growth leads to elongated rods. It is thus possible to adjust
the aspect ratio of rutile nanoparticles from around 5:1 to 15:1.
When TiCl is thermolyzed in concentrated hydrochloric acid (HCl 1–5M),
4
brookite nanoplatelets are stabilized and it is possible to obtain them as
the main product when the stoichiometries of Cl/Ti and H /Ti are opti-
mized (Figure 3.8) [37]. Brookite is currently obtained in hydrothermal
conditions at elevated temperature in the form of large particles [38].
Nanoparticles of brookite apparently are never obtained except as by-
products of various reactions [39]. Quasi-quantitative synthesis of brookite
Cl (H O) ,
nanoparticles seems to result from a specific precursor, Ti(OH) 2 2 2 2