Page 58 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 58
44 Principles and Methods
containing chloride as ligands in the early complexes formed in solution.
It has been proposed [37] that chloride ligands orient the early stages of
condensation in the formation of brookite. As long as chloride ions are pres-
ent in suspension, brookite nanoparticles remain stable, while if chloride
ions are replaced by perchloric anions, brookite transformation into rutile
is complete after several hours at 90 C.
These examples underscore the versatility of oxide nanoparticle chem-
istry in an aqueous medium. The main parameter allowing the control
of nanoparticle morphology (size, crystalline structure) is the acidity of
the reaction bath. A strict control is consequently critical to obtain well-
defined nanoparticles. It is however interesting to distinguish two sorts
of physico-chemical conditions in these syntheses.
In moderately acidic or basic media, the sign and the density of the elec-
trostatic surface charge of particles varies as a function of pH due to
proton adsorption-desorption equilibria. This involves a change in chem-
ical composition of the surface and therefore a change in surface energy
of the particle during formation. When the surface charge density is
high (the pH is far from the surface point of zero charge), the surface
energy is strongly decreased. As a consequence, the size of nanoparticles
decreases because the energetic penalty to develop surface is notably
reduced. Asemiquantitative model [28] works well to account for this size
effect for anatase and magnetite and to explain the change in shape of
(a) (b)
0.34 nm
0.34 nm 5 nm 0.34 nm 3 nm
(c) 25 nm
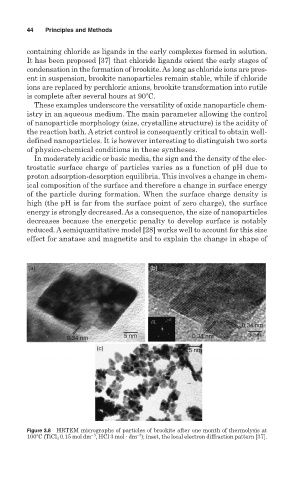
Figure 3.8 HRTEM micrographs of particles of brookite after one month of thermolysis at
3
3
100 C (TiCl 4 0.15 mol dm , HCl 3 mol dm ); inset, the local electron diffraction pattern [37].