Page 56 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 56
42 Principles and Methods
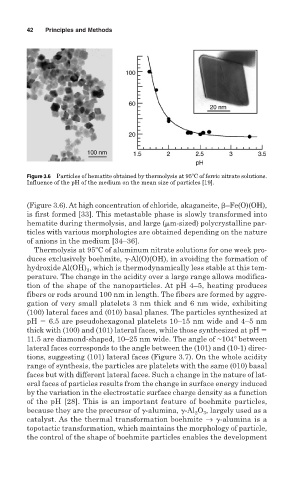
Figure 3.6 Particles of hematite obtained by thermolysis at 95 C of ferric nitrate solutions.
Influence of the pH of the medium on the mean size of particles [19].
(Figure 3.6). At high concentration of chloride, akaganeite, –Fe(O)(OH),
is first formed [33]. This metastable phase is slowly transformed into
hematite during thermolysis, and large (
m-sized) polycrystalline par-
ticles with various morphologies are obtained depending on the nature
of anions in the medium [34–36].
Thermolysis at 95 C of aluminum nitrate solutions for one week pro-
duces exclusively boehmite, -Al(O)(OH), in avoiding the formation of
hydroxide Al(OH) 3 , which is thermodynamically less stable at this tem-
perature. The change in the acidity over a large range allows modifica-
tion of the shape of the nanoparticles. At pH 4–5, heating produces
fibers or rods around 100 nm in length. The fibers are formed by aggre-
gation of very small platelets 3 nm thick and 6 nm wide, exhibiting
(100) lateral faces and (010) basal planes. The particles synthesized at
pH 6.5 are pseudohexagonal platelets 10–15 nm wide and 4–5 nm
thick with (100) and (101) lateral faces, while those synthesized at pH
11.5 are diamond-shaped, 10–25 nm wide. The angle of ~104 between
lateral faces corresponds to the angle between the (101) and (10-1) direc-
tions, suggesting (101) lateral faces (Figure 3.7). On the whole acidity
range of synthesis, the particles are platelets with the same (010) basal
faces but with different lateral faces. Such a change in the nature of lat-
eral faces of particles results from the change in surface energy induced
by the variation in the electrostatic surface charge density as a function
of the pH [28]. This is an important feature of boehmite particles,
because they are the precursor of -alumina, -Al 2 O 3 , largely used as a
catalyst. As the thermal transformation boehmite → -alumina is a
topotactic transformation, which maintains the morphology of particle,
the control of the shape of boehmite particles enables the development