Page 54 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 54
40 Principles and Methods
2 3
role of electron mobility between Fe and Fe ions in the crystal-
lization process. With other divalent cations, intervalence transfers
are negligible and a spinel ferrite forms only by dissolution-
crystallization [24]. With x 0.66, corresponding to stoichiometric
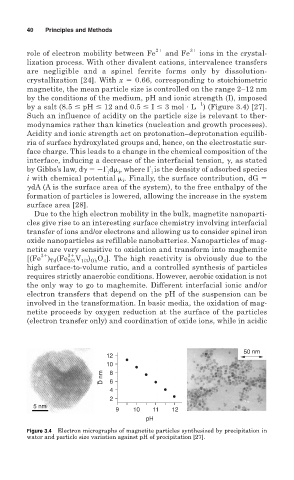
magnetite, the mean particle size is controlled on the range 2–12 nm
by the conditions of the medium, pH and ionic strength (I), imposed
1
by a salt (8.5 pH 12 and 0.5 I 3 mol L ) (Figure 3.4) [27].
Such an influence of acidity on the particle size is relevant to ther-
modynamics rather than kinetics (nucleation and growth processes).
Acidity and ionic strength act on protonation–deprotonation equilib-
ria of surface hydroxylated groups and, hence, on the electrostatic sur-
face charge. This leads to a change in the chemical composition of the
interface, inducing a decrease of the interfacial tension, , as stated
by Gibbs’s law, d d
i , where i is the density of adsorbed species
i
i with chemical potential
i . Finally, the surface contribution, dG
dA (A is the surface area of the system), to the free enthalpy of the
formation of particles is lowered, allowing the increase in the system
surface area [28].
Due to the high electron mobility in the bulk, magnetite nanoparti-
cles give rise to an interesting surface chemistry involving interfacial
transfer of ions and/or electrons and allowing us to consider spinel iron
oxide nanoparticles as refillable nanobatteries. Nanoparticles of mag-
netite are very sensitive to oxidation and transform into maghemite
3 3
[(Fe ) Td (Fe 5/3 V 1/3 ) Oh O 4 ]. The high reactivity is obviously due to the
high surface-to-volume ratio, and a controlled synthesis of particles
requires strictly anaerobic conditions. However, aerobic oxidation is not
the only way to go to maghemite. Different interfacial ionic and/or
electron transfers that depend on the pH of the suspension can be
involved in the transformation. In basic media, the oxidation of mag-
netite proceeds by oxygen reduction at the surface of the particles
(electron transfer only) and coordination of oxide ions, while in acidic
50 nm
12
10
D nm 8 6
4
2
5 nm
9 10 11 12
pH
Figure 3.4 Electron micrographs of magnetite particles synthesized by precipitation in
water and particle size variation against pH of precipitation [27].