Page 60 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 60
46 Principles and Methods
TABLE 3.1 Gelation Time of Silicon Alkoxides as a Function of Alkoxy Groups
Si(OR) 4 at Several Values of pH in Water and in 4-(Dimethylamino)Pyridine (DMAP)
Gel Time (h) at RT
Alkoxide pH 7 pH 1 (HCl, HNO 3 ) pH 9 (NH 3 ) DMAP
44
Si(OMe) 4
242 10 10 5 min
Si(OEt) 4
550
Si(OBu) 4
Alkoxides of low electronegative elements have to be handled with care,
under dry atmosphere, because traces of water can be enough to provoke
precipitation. By comparison, alkoxides of very electronegative elements
such as O P(OEt) ( 2.11) are quite inert and do not react with
3
P
water in normal conditions.
The reactivity of metal alkoxides is also very sensitive to the steric hin-
drance of the alkoxy groups. It strongly decreases when the size of the
OR group increases. For instance, the rate constant, k, for hydrolysis of
1 1
Si(OR) at 20 C decreases from 5.1 10 2 L mol s for Si(OMe) to
4
4
1 1
0.8 10 2 L mol s for Si(OBu) and the gelation time is increased
4
by a factor of 10 (Table 3.1).
The acidity of the medium also influences the rate of hydrolysis and
condensation reaction to a great extent as well as the morphology of the
products. In an excess of water and in acidic medium (pH 4), the sil-
icon alkoxides form transparent polymeric gels while in basic medium
(pH
8); the condensation is also accelerated relatively to the reaction
in neutral medium (Table 3.1) and leads to perfectly spherical and
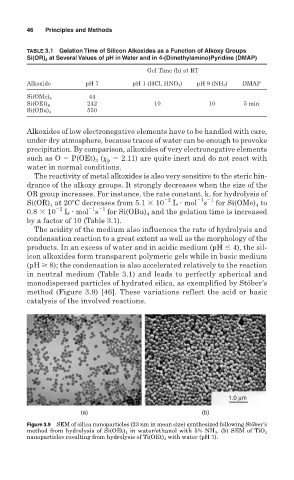
monodispersed particles of hydrated silica, as exemplified by Stöber’s
method (Figure 3.9) [46]. These variations reflect the acid or basic
catalysis of the involved reactions.
1.0 µm
(a) (b)
Figure 3.9 SEM of silica nanoparticles (23 nm in mean size) synthesized following Stöber’s
method from hydrolysis of Si(OEt) 4 in water/ethanol with 5% NH 3 . (b) SEM of TiO 2
nanoparticles resulting from hydrolysis of Ti(OEt) 4 with water (pH 7).