Page 65 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 65
Nanomaterials Fabrication 51
a 001 b
001
001
101 101 001
101
101
c
001
001
101
101
101 101
d
001
001 101 001
101
011 011
Seed
e
001
001
101
101
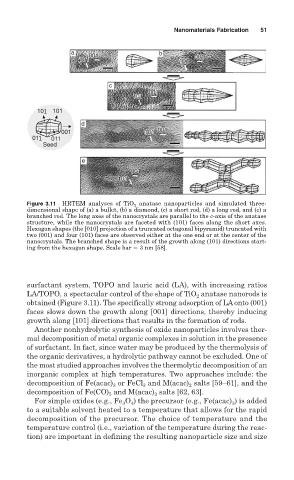
Figure 3.11 HRTEM analyses of TiO 2 anatase nanoparticles and simulated three-
dimensional shape of (a) a bullet, (b) a diamond, (c) a short rod, (d) a long rod, and (e) a
branched rod. The long axes of the nanocrystals are parallel to the c-axis of the anatase
structure, while the nanocrystals are faceted with (101) faces along the short axes.
Hexagon shapes (the [010] projection of a truncated octagonal bipyramid) truncated with
two (001) and four (101) faces are observed either at the one end or at the center of the
nanocrystals. The branched shape is a result of the growth along (101) directions start-
ing from the hexagon shape. Scale bar 3 nm [58].
surfactant system, TOPO and lauric acid (LA), with increasing ratios
LA/TOPO, a spectacular control of the shape of TiO anatase nanorods is
2
obtained (Figure 3.11). The specifically strong adsorption of LAonto (001)
faces slows down the growth along [001] directions, thereby inducing
growth along [101] directions that results in the formation of rods.
Another nonhydrolytic synthesis of oxide nanoparticles involves ther-
mal decomposition of metal organic complexes in solution in the presence
of surfactant. In fact, since water may be produced by the thermolysis of
the organic derivatives, a hydrolytic pathway cannot be excluded. One of
the most studied approaches involves the thermolytic decomposition of an
inorganic complex at high temperatures. Two approaches include: the
decomposition of Fe(acac) or FeCl and M(acac) salts [59–61], and the
3
3
2
decomposition of Fe(CO) and M(acac) salts [62, 63].
2
5
For simple oxides (e.g., Fe O ) the precursor (e.g., Fe(acac) ) is added
3
4
3
to a suitable solvent heated to a temperature that allows for the rapid
decomposition of the precursor. The choice of temperature and the
temperature control (i.e., variation of the temperature during the reac-
tion) are important in defining the resulting nanoparticle size and size