Page 64 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 64
50 Principles and Methods
+ HX
RO-MX 3
(a)
H X X
ROH
MX 4 O M X
R
X
(b)
HO-MX + RX
3
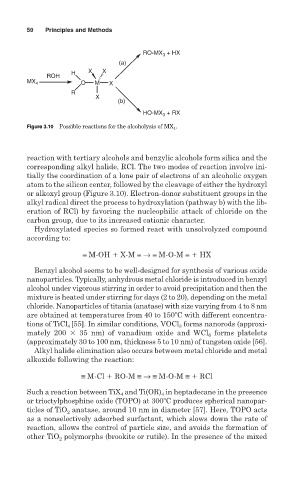
Figure 3.10 Possible reactions for the alcoholysis of MX 4 .
reaction with tertiary alcohols and benzylic alcohols form silica and the
corresponding alkyl halide, RCl. The two modes of reaction involve ini-
tially the coordination of a lone pair of electrons of an alcoholic oxygen
atom to the silicon center, followed by the cleavage of either the hydroxyl
or alkoxyl group (Figure 3.10). Electron-donor substituent groups in the
alkyl radical direct the process to hydroxylation (pathway b) with the lib-
eration of RCl) by favoring the nucleophilic attack of chloride on the
carbon group, due to its increased cationic character.
Hydroxylated species so formed react with unsolvolyzed compound
according to:
≡ M-OH X-M ≡→≡ M-O-M ≡ HX
Benzyl alcohol seems to be well-designed for synthesis of various oxide
nanoparticles. Typically, anhydrous metal chloride is introduced in benzyl
alcohol under vigorous stirring in order to avoid precipitation and then the
mixture is heated under stirring for days (2 to 20), depending on the metal
chloride. Nanoparticles of titania (anatase) with size varying from 4 to 8 nm
are obtained at temperatures from 40 to 150 C with different concentra-
tions of TiCl [55]. In similar conditions, VOCl forms nanorods (approxi-
4
3
mately 200 35 nm) of vanadium oxide and WCl 6 forms platelets
(approximately 30 to 100 nm, thickness 5 to 10 nm) of tungsten oxide [56].
Alkyl halide elimination also occurs between metal chloride and metal
alkoxide following the reaction:
≡ M-Cl RO-M ≡→≡ M-O-M ≡ RCl
Such a reaction between TiX and Ti(OR) in heptadecane in the presence
4
4
or trioctylphosphine oxide (TOPO) at 300 C produces spherical nanopar-
ticles of TiO anatase, around 10 nm in diameter [57]. Here, TOPO acts
2
as a nonselectively adsorbed surfactant, which slows down the rate of
reaction, allows the control of particle size, and avoids the formation of
other TiO polymorphs (brookite or rutile). In the presence of the mixed
2