Page 70 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 70
56 Principles and Methods
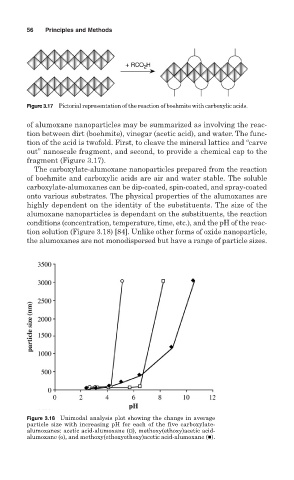
Figure 3.17 Pictorial representation of the reaction of boehmite with carboxylic acids.
of alumoxane nanoparticles may be summarized as involving the reac-
tion between dirt (boehmite), vinegar (acetic acid), and water. The func-
tion of the acid is twofold. First, to cleave the mineral lattice and “carve
out” nanoscale fragment, and second, to provide a chemical cap to the
fragment (Figure 3.17).
The carboxylate-alumoxane nanoparticles prepared from the reaction
of boehmite and carboxylic acids are air and water stable. The soluble
carboxylate-alumoxanes can be dip-coated, spin-coated, and spray-coated
onto various substrates. The physical properties of the alumoxanes are
highly dependent on the identity of the substituents. The size of the
alumoxane nanoparticles is dependant on the substituents, the reaction
conditions (concentration, temperature, time, etc.), and the pH of the reac-
tion solution (Figure 3.18) [84]. Unlike other forms of oxide nanoparticle,
the alumoxanes are not monodispersed but have a range of particle sizes.
Figure 3.18 Unimodal analysis plot showing the change in average
particle size with increasing pH for each of the five carboxylate-
alumoxanes: acetic acid-alumoxane ( ), methoxy(ethoxy)acetic acid-
alumoxane (o), and methoxy(ethoxyethoxy)acetic acid-alumoxane ( ).