Page 75 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 75
Nanomaterials Fabrication 61
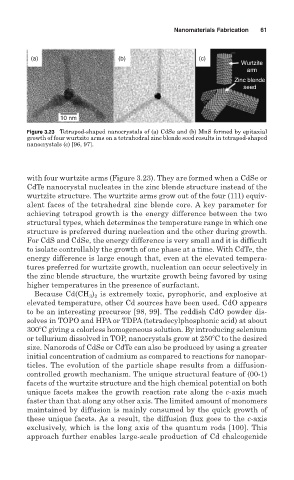
(a) (b) (c)
Wurtzite
arm
Zinc blende
seed
10 nm
Figure 3.23 Tetrapod-shaped nanocrystals of (a) CdSe and (b) MnS formed by epitaxial
growth of four wurtzite arms on a tetrahedral zinc blende seed results in tetrapod-shaped
nanocrystals (c) [96, 97].
with four wurtzite arms (Figure 3.23). They are formed when a CdSe or
CdTe nanocrystal nucleates in the zinc blende structure instead of the
wurtzite structure. The wurtzite arms grow out of the four (111) equiv-
alent faces of the tetrahedral zinc blende core. A key parameter for
achieving tetrapod growth is the energy difference between the two
structural types, which determines the temperature range in which one
structure is preferred during nucleation and the other during growth.
For CdS and CdSe, the energy difference is very small and it is difficult
to isolate controllably the growth of one phase at a time. With CdTe, the
energy difference is large enough that, even at the elevated tempera-
tures preferred for wurtzite growth, nucleation can occur selectively in
the zinc blende structure, the wurtzite growth being favored by using
higher temperatures in the presence of surfactant.
Because Cd(CH ) is extremely toxic, pyrophoric, and explosive at
3 2
elevated temperature, other Cd sources have been used. CdO appears
to be an interesting precursor [98, 99]. The reddish CdO powder dis-
solves in TOPO and HPA or TDPA (tetradecylphosphonic acid) at about
300 C giving a colorless homogeneous solution. By introducing selenium
or tellurium dissolved in TOP, nanocrystals grow at 250 C to the desired
size. Nanorods of CdSe or CdTe can also be produced by using a greater
initial concentration of cadmium as compared to reactions for nanopar-
ticles. The evolution of the particle shape results from a diffusion-
controlled growth mechanism. The unique structural feature of (00-1)
facets of the wurtzite structure and the high chemical potential on both
unique facets makes the growth reaction rate along the c-axis much
faster than that along any other axis. The limited amount of monomers
maintained by diffusion is mainly consumed by the quick growth of
these unique facets. As a result, the diffusion flux goes to the c-axis
exclusively, which is the long axis of the quantum rods [100]. This
approach further enables large-scale production of Cd chalcogenide