Page 71 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 71
Nanomaterials Fabrication 57
C C
O O O O
O O O O
Al Al Al + M(acac) n Al Al Al
O O OH O O OH
O O OH – Al(acac) 3 O O OH
Al Al Al M
O OH O OH
OH OH
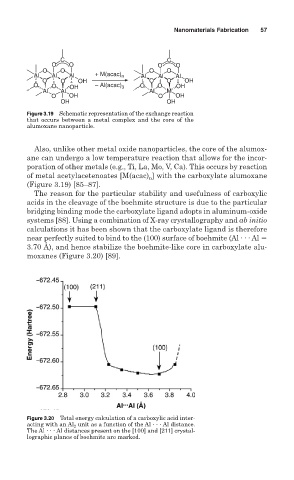
Figure 3.19 Schematic representation of the exchange reaction
that occurs between a metal complex and the core of the
alumoxane nanoparticle.
Also, unlike other metal oxide nanoparticles, the core of the alumox-
ane can undergo a low temperature reaction that allows for the incor-
poration of other metals (e.g., Ti, La, Mo, V, Ca). This occurs by reaction
of metal acetylacetenoates [M(acac) ] with the carboxylate alumoxane
n
(Figure 3.19) [85–87].
The reason for the particular stability and usefulness of carboxylic
acids in the cleavage of the boehmite structure is due to the particular
bridging binding mode the carboxylate ligand adopts in aluminum-oxide
systems [88]. Using a combination of X-ray crystallography and ab initio
calculations it has been shown that the carboxylate ligand is therefore
near perfectly suited to bind to the (100) surface of boehmite (Al . . . Al
3.70 Å), and hence stabilize the boehmite-like core in carboxylate alu-
moxanes (Figure 3.20) [89].
Figure 3.20 Total energy calculation of a carboxylic acid inter-
acting with an Al 2 unit as a function of the Al Al distance.
The Al Al distances present on the [100] and [211] crystal-
lographic planes of boehmite are marked.