Page 82 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 82
68 Principles and Methods
20 nm when the concentration of chloroauric acid decreases [119].
Here, citrate acts as a reducing agent and also as a stabilizer. Organic
ligands, with a group such as phosphine, having a strong affinity for
metal are often used to stabilize metallic nanoparticles. For gold, thiol
derivatives are strong stabilizers and the reduction of Au(III) ions by
citrate or borohydride in the presence of a thiol ligand gives uniform
Au nanoparticles, the Au/thiol ratio controlling the mean size of the
nanoparticles [120]. Silver nanoparticles are similarly obtained by
reduction of silver nitrate by ferrous citrate in an aqueous medium,
their stabilization in solution resulting from silver citrate adsorption
[121]. Very uniform silver nanoparticles have also been obtained by
reduction in nonaqueous media [122]. Aqueous AgNO was vigorously
3
mixed with chloroform containing tetra n-octylammonium bromide,
[(C H ) N]Br, acting as a catalyst for phase transfer. 1-nonanethiol
8
17 4
was first added to the gray organic phase collected followed by an aque-
ous solution of sodium borohydride (NaBH ). A stable dispersion of
4
nearly spherical 1-nonanethiol–capped silver nanoparticles in chloro-
form was obtained. The evaporation of the solvent onto a carbon-coated
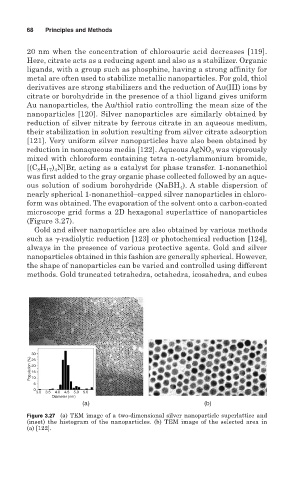
microscope grid forms a 2D hexagonal superlattice of nanoparticles
(Figure 3.27).
Gold and silver nanoparticles are also obtained by various methods
such as -radiolytic reduction [123] or photochemical reduction [124],
always in the presence of various protective agents. Gold and silver
nanoparticles obtained in this fashion are generally spherical. However,
the shape of nanoparticles can be varied and controlled using different
methods. Gold truncated tetrahedra, octahedra, icosahedra, and cubes
30
Population (%) 20
25
15
10
5
0
3.0 3.5 4.0 4.5 5.0 5.0
Diameter (nm)
(a) (b)
Figure 3.27 (a) TEM image of a two-dimensional silver nanoparticle superlattice and
(inset) the histogram of the nanoparticles. (b) TEM image of the selected area in
(a) [122].