Page 123 - Essentials of physical chemistry
P. 123
The Second and Third Laws of Thermodynamics 85
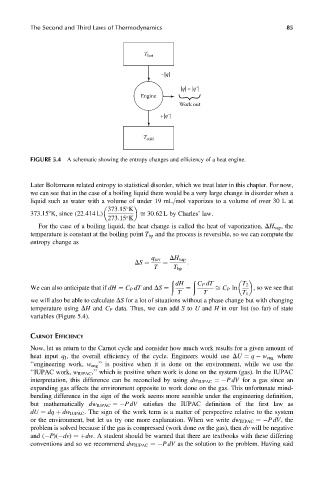
T hot
–|q|
|q|– |q΄ |
Engine
Work out
+|q΄ |
T cold
FIGURE 5.4 A schematic showing the entropy changes and efficiency of a heat engine.
Later Boltzmann related entropy to statistical disorder, which we treat later in this chapter. For now,
we can see that in the case of a boiling liquid there would be a very large change in disorder when a
liquid such as water with a volume of under 19 mL=mol vaporizes to a volume of over 30 L at
373:15 K
373.158K, since (22:414 L) ffi 30:62 L by Charles’ law.
273:15 K
For the case of a boiling liquid, the heat change is called the heat of vaporization, DH vap , the
temperature is constant at the boiling point T bp and the process is reversible, so we can compute the
entropy change as
q rev DH vap
:
DS ¼ ¼
T T bp
ð ð
dH C P dT T 2
ffi C P ln , so we see that
We can also anticipate that if dH ¼ C P dT and DS ¼ ¼
T T T 1
we will also be able to calculate DS for a lot of situations without a phase change but with changing
temperature using DH and C P data. Thus, we can add S to U and H in our list (so far) of state
variables (Figure 5.4).
CARNOT EFFICIENCY
Now, let us return to the Carnot cycle and consider how much work results for a given amount of
heat input q I , the overall efficiency of the cycle. Engineers would use DU ¼ q w eng where
‘‘engineering work, w eng ’’ is positive when it is done on the environment, while we use the
‘‘IUPAC work, w IUPAC ,’’ which is positive when work is done on the system (gas). In the IUPAC
interpretation, this difference can be reconciled by using dw IUPAC ¼ PdV for a gas since an
expanding gas affects the environment opposite to work done on the gas. This unfortunate mind-
bending difference in the sign of the work seems more sensible under the engineering definition,
but mathematically dw IUPAC ¼ PdV satisfies the IUPAC definition of the first law as
dU ¼ dq þ dw IUPAC . The sign of the work term is a matter of perspective relative to the system
or the environment, but let us try one more explanation. When we write dw IUPAC ¼ PdV, the
problem is solved because if the gas is compressed (work done on the gas), then dv will be negative
and ( P)( dv) ¼þdw. A student should be warned that there are textbooks with these differing
conventions and so we recommend dw IUPAC ¼ PdV as the solution to the problem. Having said