Page 188 - Essentials of physical chemistry
P. 188
150 Essentials of Physical Chemistry
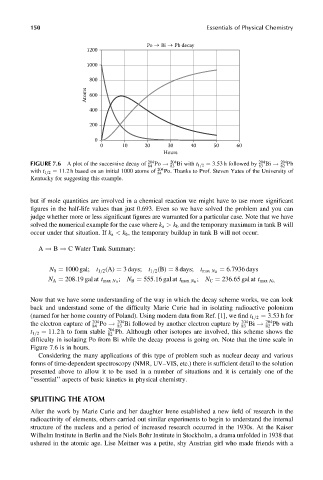
Po Bi Pb decay
1200
1000
800
Atoms 600
400
200
0
0 10 20 30 40 50 60
Hours
FIGURE 7.6 A plot of the successive decay of 204 Po ! 204 Bi with t 1=2 ¼ 3:53 h followed by 204 Bi ! 204 Pb
84
83
83
82
with t 1=2 ¼ 11:2 h based on an initial 1000 atoms of 204 Po. Thanks to Prof. Steven Yates of the University of
84
Kentucky for suggesting this example.
but if mole quantities are involved in a chemical reaction we might have to use more significant
figures in the half-life values than just 0.693. Even so we have solved the problem and you can
judge whether more or less significant figures are warranted for a particular case. Note that we have
solved the numerical example for the case where k a > k b and the temporary maximum in tank B will
occur under that situation. If k a < k b , the temporary buildup in tank B will not occur.
A ! B ! C Water Tank Summary:
N 0 ¼ 1000 gal; t 1=2 (A) ¼ 3 days; t 1=2 (B) ¼ 8 days; t max N B ¼ 6:7936 days
; ;
N A ¼ 208:19 gal at t max N B N B ¼ 555:16 gal at t max N B N C ¼ 236:65 gal at t max N B
Now that we have some understanding of the way in which the decay scheme works, we can look
back and understand some of the difficulty Marie Curie had in isolating radioactive polonium
(named for her home country of Poland). Using modern data from Ref. [1], we find t 1=2 ¼ 3:53 h for
the electron capture of 204 204 Bi followed by another electron capture by 204 204 Pb with
84 Po ! 83 83 Bi ! 82
t 1=2 ¼ 11:2 h to form stable 204 Pb. Although other isotopes are involved, this scheme shows the
82
difficulty in isolating Po from Bi while the decay process is going on. Note that the time scale in
Figure 7.6 is in hours.
Considering the many applications of this type of problem such as nuclear decay and various
forms of time-dependent spectroscopy (NMR, UV–VIS, etc.) there is sufficient detail to the solution
presented above to allow it to be used in a number of situations and it is certainly one of the
‘‘essential’’ aspects of basic kinetics in physical chemistry.
SPLITTING THE ATOM
After the work by Marie Curie and her daughter Irene established a new field of research in the
radioactivity of elements, others carried out similar experiments to begin to understand the internal
structure of the nucleus and a period of increased research occurred in the 1930s. At the Kaiser
Wilhelm Institute in Berlin and the Niels Bohr Institute in Stockholm, a drama unfolded in 1938 that
ushered in the atomic age. Lise Meitner was a petite, shy Austrian girl who made friends with a