Page 221 - Essentials of physical chemistry
P. 221
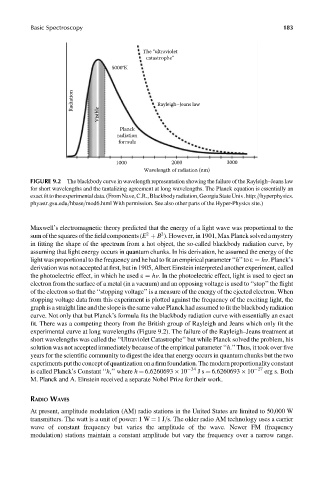
Basic Spectroscopy 183
The “ultraviolet
catastrophe”
5000°K
Radiation Rayleigh–Jeans law
Visible
Planck
radiation
formula
1000 2000 3000
Wavelength of radiation (nm)
FIGURE 9.2 The blackbody curve in wavelength representation showing the failure of the Rayleigh–Jeans law
for short wavelengths and the tantalizing agreement at long wavelengths. The Planck equation is essentially an
exactfittotheexperimentaldata.(FromNave,C.R.,Blackbodyradiation,GeorgiaStateUniv.http:==hyperphysics.
phyastr.gsu.edu=hbase=mod6.html With permission. See also other parts of the Hyper-Physics site.)
Maxwell’s electromagnetic theory predicted that the energy of a light wave was proportional to the
2
2
sum of the squares of the field components (E þ B ). However, in 1901, Max Planck solved a mystery
in fitting the shape of the spectrum from a hot object, the so-called blackbody radiation curve, by
assuming that light energy occurs in quantum chunks. In his derivation, he assumed the energy of the
light was proportional to the frequency and he had to fit an empirical parameter ‘‘h’’ to e ¼ hn. Planck’s
derivation was not accepted at first, but in 1905, Albert Einstein interpreted another experiment, called
the photoelectric effect, in which he used e ¼ hn. In the photoelectric effect, light is used to eject an
electron from the surface of a metal (in a vacuum) and an opposing voltage is used to ‘‘stop’’ the flight
of the electron so that the ‘‘stopping voltage’’ is a measure of the energy of the ejected electron. When
stopping voltage data from this experiment is plotted against the frequency of the exciting light, the
graph is a straight line and the slope is the same value Planck had assumed to fit the blackbody radiation
curve. Not only that but Planck’s formula fits the blackbody radiation curve with essentially an exact
fit. There was a competing theory from the British group of Rayleigh and Jeans which only fit the
experimental curve at long wavelengths (Figure 9.2). The failure of the Rayleigh–Jeans treatment at
short wavelengths was called the ‘‘Ultraviolet Catastrophe’’ but while Planck solved the problem, his
solution was not accepted immediately because of the empirical parameter ‘‘h.’’ Thus, it took over five
years for the scientific community to digest the idea that energy occurs in quantum chunks but the two
experiments put the concept of quantization ona firm foundation. The modern proportionality constant
is called Planck’s Constant ‘‘h,’’ where h ¼ 6.6260693 10 34 Js ¼ 6.6260693 10 27 erg s. Both
M. Planck and A. Einstein received a separate Nobel Prize for their work.
RADIO WAVES
At present, amplitude modulation (AM) radio stations in the United States are limited to 50,000 W
transmitters. The watt is a unit of power: 1 W ¼ 1J=s. The older radio AM technology uses a carrier
wave of constant frequency but varies the amplitude of the wave. Newer FM (frequency
modulation) stations maintain a constant amplitude but vary the frequency over a narrow range.