Page 222 - Essentials of physical chemistry
P. 222
184 Essentials of Physical Chemistry
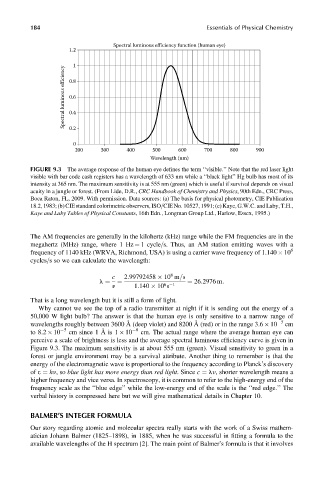
Spectral luminous efficiency function (human eye)
1.2
1
Spectral luminous efficiency 0.8
0.6
0.4
0.2
0
200 300 400 500 600 700 800 900
Wavelength (nm)
FIGURE 9.3 The average response of the human eye defines the term ‘‘visible.’’ Note that the red laser light
visible with bar code cash registers has a wavelength of 633 nm while a ‘‘black light’’ Hg bulb has most of its
intensity at 365 nm. The maximum sensitivity is at 555 nm (green) which is useful if survival depends on visual
acuity in a jungle or forest. (From Lide, D.R., CRC Handbook of Chemistry and Physics, 90th Edn., CRC Press,
Boca Raton, FL, 2009. With permission. Data sources: (a) The basis for physical photometry, CIE Publication
18.2,1983; (b) CIE standard colorimetric observers, ISO=CIE No. 10527, 1991;(c) Kaye,G.W.C. andLaby,T.H.,
Kaye and Laby Tables of Physical Constants, 16th Edn., Longman Group Ltd., Harlow, Essex, 1995.)
The AM frequencies are generally in the kilohertz (kHz) range while the FM frequencies are in the
megahertz (MHz) range, where 1 Hz ¼ 1 cycle=s. Thus, an AM station emitting waves with a
frequency of 1140 kHz (WRVA, Richmond, USA) is using a carrier wave frequency of 1.140 10 6
cycles=s so we can calculate the wavelength:
8
c 2:99792458 10 m=s
¼ 26:2976 m:
n 1:140 10 s
l ¼ ¼ 6 1
That is a long wavelength but it is still a form of light.
Why cannot we see the top of a radio transmitter at night if it is sending out the energy of a
50,000 W light bulb? The answer is that the human eye is only sensitive to a narrow range of
wavelengths roughly between 3600 Å (deep violet) and 8200 Å (red) or in the range 3.6 10 5 cm
to 8.2 10 5 cm since 1 Å is 1 10 8 cm. The actual range where the average human eye can
perceive a scale of brightness is less and the average spectral luminous efficiency curve is given in
Figure 9.3. The maximum sensitivity is at about 555 nm (green). Visual sensitivity to green in a
forest or jungle environment may be a survival attribute. Another thing to remember is that the
energy of the electromagnetic wave is proportional to the frequency according to Planck’s discovery
of e ¼ hn,so blue light has more energy than red light. Since c ¼ ln, shorter wavelength means a
higher frequency and vice versa. In spectroscopy, it is common to refer to the high-energy end of the
frequency scale as the ‘‘blue edge’’ while the low-energy end of the scale is the ‘‘red edge.’’ The
verbal history is compressed here but we will give mathematical details in Chapter 10.
BALMER’S INTEGER FORMULA
Our story regarding atomic and molecular spectra really starts with the work of a Swiss mathem-
atician Johann Balmer (1825–1898), in 1885, when he was successful in fitting a formula to the
available wavelengths of the H spectrum [2]. The main point of Balmer’s formula is that it involves