Page 73 - Flexible Robotics in Medicine
P. 73
Cable-driven flexible endoscope utilizing diamond-shaped perforations: FlexDiamond 57
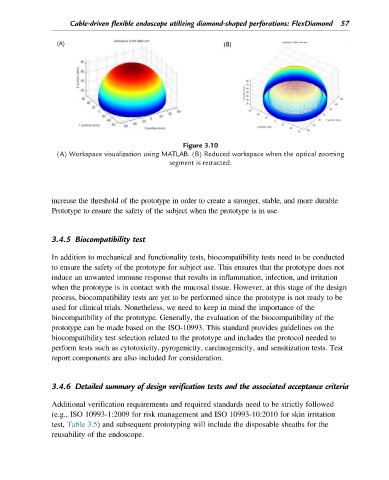
Figure 3.10
(A) Workspace visualization using MATLAB. (B) Reduced workspace when the optical zooming
segment is retracted.
increase the threshold of the prototype in order to create a stronger, stable, and more durable
Prototype to ensure the safety of the subject when the prototype is in use.
3.4.5 Biocompatibility test
In addition to mechanical and functionality tests, biocompatibility tests need to be conducted
to ensure the safety of the prototype for subject use. This ensures that the prototype does not
induce an unwanted immune response that results in inflammation, infection, and irritation
when the prototype is in contact with the mucosal tissue. However, at this stage of the design
process, biocompatibility tests are yet to be performed since the prototype is not ready to be
used for clinical trials. Nonetheless, we need to keep in mind the importance of the
biocompatibility of the prototype. Generally, the evaluation of the biocompatibility of the
prototype can be made based on the ISO-10993. This standard provides guidelines on the
biocompatibility test selection related to the prototype and includes the protocol needed to
perform tests such as cytotoxicity, pyrogenicity, carcinogenicity, and sensitization tests. Test
report components are also included for consideration.
3.4.6 Detailed summary of design verification tests and the associated acceptance criteria
Additional verification requirements and required standards need to be strictly followed
(e.g., ISO 10993-1:2009 for risk management and ISO 10993-10:2010 for skin irritation
test, Table 3.5) and subsequent prototyping will include the disposable sheaths for the
reusability of the endoscope.