Page 163 - Fluid Catalytic Cracking Handbook
P. 163
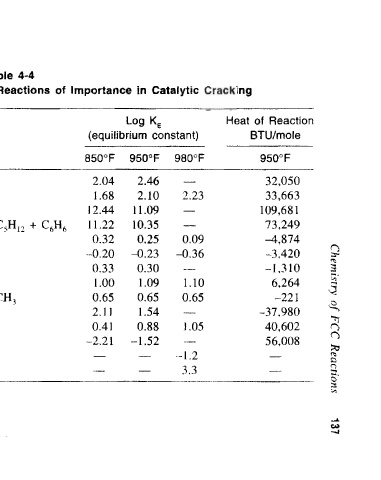
Cracking
Cyclization
Dealkylation
Isomerization
Polymerization
Source: Venuto [2]
Transalkylation
Reaction Class
Dehydrogenation
Hydrogen transfer
Paraffin Alkylation
3C 2H 4 — > 1-C 6H 12
1~C 8H 16 -> 2C 4H g
n-C 6H 10 -» iso-C 4H 10
1-C 4H 8 -» trans-2-C 4H 8
4C 6H 12 -» 3C 6H 14 + C 6H 6
n-C 6H 14 ^ 1-C 6H 12 + H 2
n-C 10H 22 -> n-C 7H 16 + C 3H 6
1-C 7H 14 -» CH 3-cyclo-C 6H 11
Specific Reaction
iso-C 3H 7-C 6H 5 -> C 6H 6 + C 3H 6
o-C 6H 4(CH 3) 2 -> m-C 6H 4(CH 3) 2
cyclo-C 6H 12 -» CH 3-cyclo-C 5H 9
1-C 4H 8 + iso-C 4H 10 -> iso-C 8H 18
C 6H 6 + m-C 6H 4(CH 3) 2 -> 2C 6H 5CH 3
Table 4-4
cyclo-C 6H l2 + 3 1-C 5H !0 -> 3n-C 5H 12 + C 6H 6
—
—
-2.21
12.44
1.00
2.11
11.22
850°F
0.32
0.33
0.65
0.41
1.68
-0.20
2.04
—
—
-1.52
0.88
0.25
10.35
11.09
1.54
1.09
0.65
0.30
950°F
-0.23
2.10
2.46
Log K E
—
—
—
-1.2
—
—
—
3.3
(equilibrium constant)
1.10
0.65
0.09
1.05
2.23
-0.36
980°F
Some Thermodynamic Data for Idealized Reactions of Importance in Catalytic Cracking
_
—
950°F
-221
6,264
-3,420
-37,980
40,602
73,249
-4,874
-1,310
56,008
32,050
33,663
109,681
BTU/moie
Heat of Reaction
O
O
O