Page 65 - Instant notes
P. 65
Entropy 51
infinitely slowly. An irreversible process involves the transfer of energy under any other
conditions. In an irreversible process energy is transferred in a manner which results in
random motion and some of the energy is dissipated as heat. The process is irreversible
because a proportion of this heat is dispersed irrecoverably, and the original conditions
cannot therefore be generated without work being done on the system.
The isothermal expansion of an ideal gas (see Topic A1) against an external pressure
is usually given to illustrate the difference between these two conditions. The work, w,
done by the gas is given by:
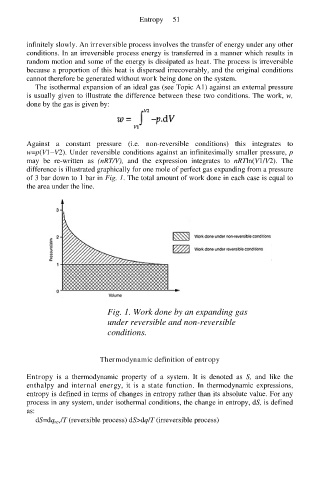
Against a constant pressure (i.e. non-reversible conditions) this integrates to
w=p(V1−V2). Under reversible conditions against an infinitesimally smaller pressure, p
may be re-written as (nRT/V), and the expression integrates to nRTln(V1/V2). The
difference is illustrated graphically for one mole of perfect gas expanding from a pressure
of 3 bar down to 1 bar in Fig. 1. The total amount of work done in each case is equal to
the area under the line.
Fig. 1. Work done by an expanding gas
under reversible and non-reversible
conditions.
Thermodynamic definition of entropy
Entropy is a thermodynamic property of a system. It is denoted as S, and like the
enthalpy and internal energy, it is a state function. In thermodynamic expressions,
entropy is defined in terms of changes in entropy rather than its absolute value. For any
process in any system, under isothermal conditions, the change in entropy, dS, is defined
as:
dS=dq rev/T (reversible process) dS>dq/T (irreversible process)