Page 70 - Instant notes
P. 70
Physical Chemistry 56
that it is either fast or random. The definition of a spontaneous process has no
implications for the rate at which it may come about—a process may be described as
spontaneous, but take an infinite amount of time to occur.
For a spontaneous process to take place, the system must be at a position where it is
ready for change to come about without the need for work to be done
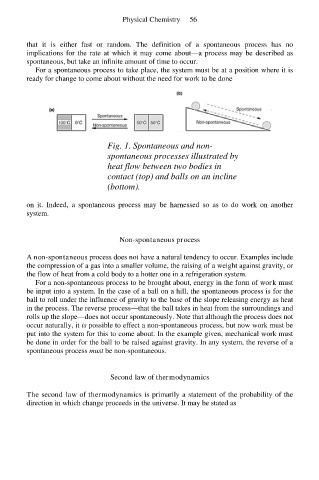
Fig. 1. Spontaneous and non-
spontaneous processes illustrated by
heat flow between two bodies in
contact (top) and balls on an incline
(bottom).
on it. Indeed, a spontaneous process may be harnessed so as to do work on another
system.
Non-spontaneous process
A non-spontaneous process does not have a natural tendency to occur. Examples include
the compression of a gas into a smaller volume, the raising of a weight against gravity, or
the flow of heat from a cold body to a hotter one in a refrigeration system.
For a non-spontaneous process to be brought about, energy in the form of work must
be input into a system. In the case of a ball on a hill, the spontaneous process is for the
ball to roll under the influence of gravity to the base of the slope releasing energy as heat
in the process. The reverse process—that the ball takes in heat from the surroundings and
rolls up the slope—does not occur spontaneously. Note that although the process does not
occur naturally, it is possible to effect a non-spontaneous process, but now work must be
put into the system for this to come about. In the example given, mechanical work must
be done in order for the ball to be raised against gravity. In any system, the reverse of a
spontaneous process must be non-spontaneous.
Second law of thermodynamics
The second law of thermodynamics is primarily a statement of the probability of the
direction in which change proceeds in the universe. It may be stated as