Page 258 - Fundamentals of Enhanced Oil and Gas Recovery
P. 258
246 Alireza Keshavarz et al.
encompass two to three times more gas compared to the same volume of a sandstone
having 25% porosity and 30% water saturation, with the same burial depth [30].
Corresponding to the unique gas-storage mechanism in coals, the estimation of
original gas in place, at preliminary stages of field development and during the pro-
duction profile, is not viable through conventional volumetric approaches for sand-
stone reservoirs. Therefore, in reserve estimation in coalbeds, a new set of equations
considering adsorption must be taken into account. Yang suggested that for a given
adsorbate and adsorbent, the amount of adsorption at equilibrium is a function of
pressure and temperature [45]
ð
V 5 Fp; TÞ (8.11)
Therefore, at constant temperature, the rate of adsorption is only a function of
pressure. Assuming negligible temperature change in the reservoir during production,
the adsorption of gaseous phase on coal rocks is described by sorption isotherms. In
fact, a sorption isotherm illustrates the relationship between the volume of adsorption
of a given adsorbate on the surface of a specific adsorptive rock as a function of pres-
sure, at a constant temperature. The adsorption of a gas on a solid has been catego-
rized into five different types, each of which has its distinct curve on the
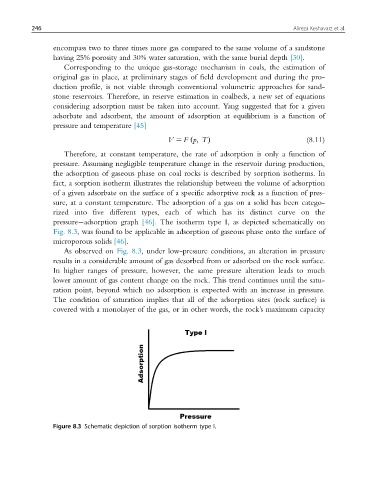
pressure adsorption graph [46]. The isotherm type I, as depicted schematically on
Fig. 8.3, was found to be applicable in adsorption of gaseous phase onto the surface of
microporous solids [46].
As observed on Fig. 8.3, under low-pressure conditions, an alteration in pressure
results in a considerable amount of gas desorbed from or adsorbed on the rock surface.
In higher ranges of pressure, however, the same pressure alteration leads to much
lower amount of gas content change on the rock. This trend continues until the satu-
ration point, beyond which no adsorption is expected with an increase in pressure.
The condition of saturation implies that all of the adsorption sites (rock surface) is
covered with a monolayer of the gas, or in other words, the rock’s maximum capacity
Figure 8.3 Schematic depiction of sorption isotherm type I.