Page 289 - Fundamentals of Gas Shale Reservoirs
P. 289
GAS STORAGE MECHANISMS 269
100 gas. Furthermore for the same porosity, a sample with smaller
pores will store more adsorbed gas.
90
In this chapter, the consideration of storage mechanisms to
Cumulative volume percentage 60 adsorbed methane on the pore walls, so that the pore space
80
Langmuir adsorption and free gas in the nanopores is restricted.
As pore pressure, P increases the volume occupied by the
70
available for free gas storage decreases. This implies that total
gas storage cannot just be taken as the sum of the adsorbed
50
methane and the amount of methane that could be stored in a
40
pore space determined by a low‐pressure helium porosity
30
adsorbs significantly less than methane (Ambrose et al., 2010;
20
Sigal et al., 2010, 2013; Sigal, 2013b). The fact that measure
6454.95C
10 6454.95S measurement, both because it is low pressure and helium
ments are usually made on ground up samples only adds to the
0 potential error. In the case of hydrocarbon liquids, the overesti
1 10 100 1000 mation of reserves could be even worse. This is because liquids
Pore diameter (nm) are less compressible so that maximum adsorption occurs at
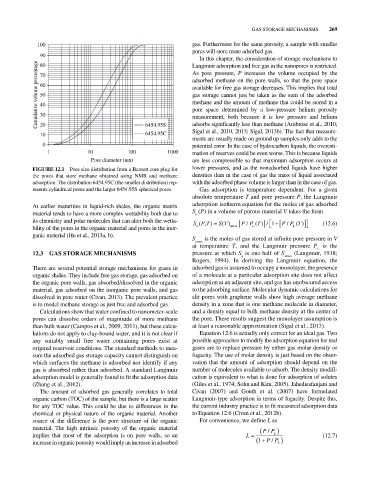
FIGURE 12.1 Pore size distribution from a Barnett core plug for lower pressures, and as the nonadsorbed liquids have higher
the pores that store methane obtained using NMR and methane densities than in the case of gas the mass of liquid associated
adsorption. The distribution 6454.95C (the smaller distribution) rep with the adsorbed phase volume is larger than in the case of gas.
resents cylindrical pores and the larger 6454.95S spherical pores. Gas adsorption is temperature dependent. For a given
absolute temperature T and pore pressure P, the Langmuir
At earlier maturities in liquid‐rich shales, the organic matrix adsorption isotherm equation for the moles of gas adsorbed
material tends to have a more complex wettability both due to S (P) in a volume of porous material V takes the form
a
its chemistry and polar molecules that can alter both the wetta SP T) S T() P PT() / 1 PP T() (12.6)
/
/
,
(
bility of the pores in the organic material and pores in the inor a amax L L
ganic material (Hu et al., 2013a, b).
S amax is the moles of gas stored at infinite pore pressure in V
at temperature T, and the Langmuir pressure P is the
L
12.3 GAS STORAGE MECHANISMS pressure at which S is one‐half of S amax (Langmuir, 1918;
a
Rogers, 1994). In deriving the Langmuir equation, the
There are several potential storage mechanisms for gases in adsorbed gas is assumed to occupy a monolayer, the presence
organic shales. They include free gas storage, gas adsorbed on of a molecule at a particular adsorption site does not affect
the organic pore walls, gas absorbed/dissolved in the organic adsorption at an adjacent site, and gas has unobscured access
material, gas adsorbed on the inorganic pore walls, and gas to the adsorbing surface. Molecular dynamic calculations for
dissolved in pore water (Civan, 2013). The prevalent practice slit pores with graphene walls show high average methane
is to model methane storage as just free and adsorbed gas. density in a zone that is one methane molecule in diameter,
Calculations show that water confined to nanometer‐scale and a density equal to bulk methane density at the center of
pores can dissolve orders of magnitude of more methane the pore. These results suggest the monolayer assumption is
than bulk water (Campos et al., 2009, 2011), but these calcu at least a reasonable approximation (Sigal et al., 2013).
lations do not apply to clay‐bound water, and it is not clear if Equation 12.6 is actually only correct for an ideal gas. Two
any suitably small free water containing pores exist at possible approaches to modify the adsorption equation for real
original reservoir conditions. The standard methods to mea gases are to replace pressure by either gas molar density or
sure the adsorbed gas storage capacity cannot distinguish on fugacity. The use of molar density is just based on the obser
which surfaces the methane is adsorbed nor identify if any vation that the amount of adsorption should depend on the
gas is absorbed rather than adsorbed. A standard Langmuir number of molecules available to adsorb. The density modifi
adsorption model is generally found to fit the adsorption data cation is equivalent to what is done for adsorption of solutes
(Zhang et al., 2012). (Giles et al., 1974; Sohn and Kim, 2005). Jahediesfanjani and
The amount of adsorbed gas generally correlates to total Civan (2007) and Gouth et al. (2007) have formulated
organic carbon (TOC) of the sample, but there is a large scatter Langmuir‐type adsorption in terms of fugacity. Despite this,
for any TOC value. This could be due to differences in the the current industry practice is to fit measured adsorption data
chemical or physical nature of the organic material. Another to Equation 12.6 (Civan et al., 2012b).
source of the difference is the pore structure of the organic For convenience, we define L as
material. The high intrinsic porosity of the organic material PP
/
implies that most of the adsorption is on pore walls, so an L L (12.7)
/
increase in organic porosity would imply an increase in adsorbed 1 PP L