Page 403 - Fundamentals of Reservoir Engineering
P. 403
IMMISCIBLE DISPLACEMENT 338
OIL
OIL
WATER
WATER
Θ Θ
(a) (b)
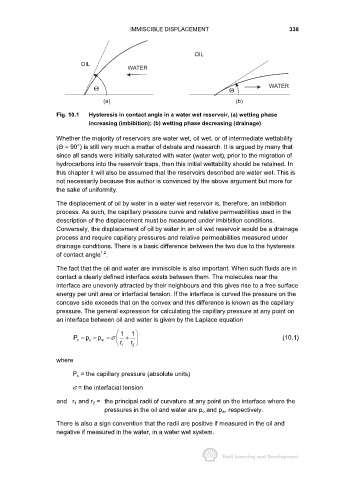
Fig. 10.1 Hysteresis in contact angle in a water wet reservoir, (a) wetting phase
increasing (imbibition); (b) wetting phase decreasing (drainage)
Whether the majority of reservoirs are water wet, oil wet, or of intermediate wettability
(Θ ≈ 90°) is still very much a matter of debate and research. It is argued by many that
since all sands were initially saturated with water (water wet), prior to the migration of
hydrocarbons into the reservoir traps, then this initial wettability should be retained. In
this chapter it will also be assumed that the reservoirs described are water wet. This is
not necessarily because this author is convinced by the above argument but more for
the sake of uniformity.
The displacement of oil by water in a water wet reservoir is, therefore, an imbibition
process. As such, the capillary pressure curve and relative permeabilities used in the
description of the displacement must be measured under imbibition conditions.
Conversely, the displacement of oil by water in an oil wet reservoir would be a drainage
process and require capillary pressures and relative permeabilities measured under
drainage conditions. There is a basic difference between the two due to the hysteresis
1,2
of contact angle .
The fact that the oil and water are immiscible is also important. When such fluids are in
contact a clearly defined interface exists between them. The molecules near the
interface are unevenly attracted by their neighbours and this gives rise to a free surface
energy per unit area or interfacial tension. If the interface is curved the pressure on the
concave side exceeds that on the convex and this difference is known as the capillary
pressure. The general expression for calculating the capillary pressure at any point on
an interface between oil and water is given by the Laplace equation
1 1
P = p − p = σ + (10.1)
w
c
o
r 1 r 2
where
P c = the capillary pressure (absolute units)
σ = the interfacial tension
and r 1 and r 2 = the principal radii of curvature at any point on the interface where the
pressures in the oil and water are p o and p w, respectively.
There is also a sign convention that the radii are positive if measured in the oil and
negative if measured in the water, in a water wet system.