Page 253 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 253
208 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
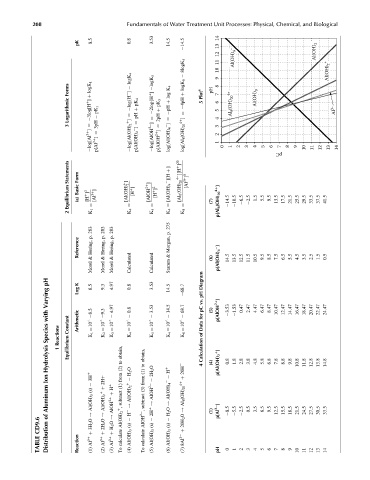
pK 8.5 0.8 3.53 14.5 14.5 1011121314 Al(OH) 2
8logK 5 Al(OH) 4 – Al(OH) 2 +
logK 4 logK 5 9
Forms logK 1 þ K 4 log logK 8 þ Plot a pH 8 7 Al(OH) 3
Logarithmic ¼ 3log[H þ ] pK 1 ¼ log{H þ ] pK 4 þ pH ¼ 2log{H þ ] pK 5 þ 2pH þ ¼ pH ¼ 4pH 5 6 5 Al 8 (OH) 20 4+ Al 3+
3 3pH ¼ ¼ ¼ 4 3
log[Al 3þ ] p[Al 3þ ] log[Al(OH) 2 þ ] p[Al(OH) 2 þ ] log[AlOH 2þ ] p[AlOH 2þ ] log[Al(OH) 4 ] log[Al 8 (OH) 20 4þ ] 2 1 0 1 2 3 4 5 6 7 pC 8 9 10 11 12 13 14
Statements Form [Al(OH) 4 ][H þ ] [Al 8 (OH) 20 4þ ][H þ ] 20 [Al 3þ ] 8
Equilibrium Basic (a) [H þ ] 3 [Al 3þ ] [Al(OH) þ ] 2 [H þ ] [AlOH 2þ ] [H þ ] 2 (7) p[Al 8 (OH) 20 4þ ] 14.5 10.5 6.5 2.5 1.5 5.5 9.5 13.5 17.5 21.5 25.5 29.5 33.5 37.5 41.5
2 ¼ K 1 ¼ K 4 ¼ K 5 ¼ K 4 ¼ K 8
275
283 283 283 p.
p. p. p.
Reference Hering, Hering, Hering, Morgan, & 9.5 8.5 7.5 6.5 5.5 4.5 3.5 2.5 1.5 0.5
Morel & & Morel & Morel Calculated Calculated Stumm (6) p[Al(OH) 4 ] 14.5 13.5 12.5 11.5 10.5
pH K 8.5 9.3 4.97 0.8 3.53 14.5 68.7 Diagram
Varying Log pH vs.
with Constant Arithmetic þ8.5 þ9.3 4.97 þ 0.8 þ 3.53 þ 14.5 68.7 pC for (5) p[AlOH 2þ ] 3.53 1.53 0.47 2.47 4.47 6.47 8.47 10.47 12.47 14.47 16.47 18.47 20.47 22.47 24.47
Species Reactions K 1 ¼ 10 ^ K 2 ¼ 10 ^ K 3 ¼ 10 ^ K 4 ¼ 10 ^ K 5 ¼ 10 ^ K 4 ¼ 10 ^ K 8 ¼ 10 ^ Data of
Hydrolysis 1 Equilibrium obtain, to (2) obtain, to Calculation 4 (4) p[Al(OH) 2 þ ] 0.8 1.8 2.8 3.8 4.8 5.8 6.8 7.8 8.8 9.8 10.8 11.8 12.8 13.8 14.8
Ion 3H þ þ 2Hþ þ H þ from (1) H 2 O þ (1) from 2H 2 O þ H þ þ 20H þ þ
Aluminum (s) Al(OH) 3 Al(OH) 2 þ þ AlOH 2þ subtract Al(OH) 2 þ ! (3) subtract AlOH 2þ ! Al(OH) 4 ! Al 8 (OH) 20 4þ
of ! ! ! Al(OH) 2 þ , H þ þ AlOH 2þ , 2H þ þ H 2 O þ ! 20H 2 O (1) p[Al 3þ ] 8.5 5.5 2.5 0.5 3.5 6.5 9.5 12.5 15.5 18.5 21.5 24.5 27.5 30.5 33.5
CD9.6 3H 2 O þ 2H 2 O þ H 2 O þ (s) (s) (s) þ
TABLE Distribution Reaction Al 3þ (1) Al 3þ (2) Al 3þ (3) calculate To Al(OH) 3 (4) calculate To Al(OH) 3 (5) Al(OH) 3 (6) 8Al 3þ (7) pH 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14