Page 250 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 250
Coagulation 205
)
Alkalinity (mg/L as CaCO ) Alkalinity (mg/L as CaCO 3
3
0 5 10 15 20 25 30 0 5 10 15 20 25 30
1.0 1.0 10
100 0.8 0.8 8
80
Alum dosage at CCC (mg/L as Al 2 (SO 4 ) 3 . 14H 2 O/L) 60 0.6 Al 3+ dosage at CCC (meq/L) Settled water turbidity (NTU) 0.6 6 4 pH
0.4
0.4
40
20
0 0.2 0.2 2
0.0 0.0 0
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.0 0.1 0.2 0.3 0.4 0.5 0.6
(a) Alkalinity (meq/L) (b) Alkalinity (meq/L)
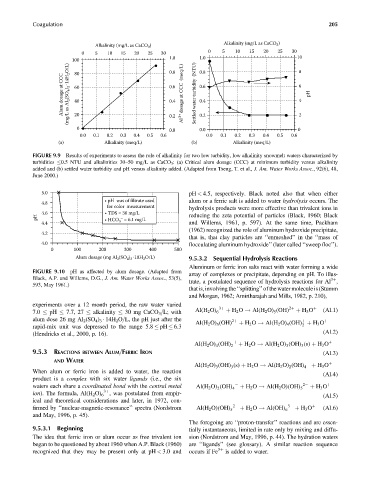
FIGURE 9.9 Results of experiments to assess the role of alkalinity for two low turbidity, low alkalinity snowmelt waters characterized by
turbidities 0.5 NTU and alkalinities 30–50 mg=L as CaCO 3 : (a) Critical alum dosage (CCC) at minimum turbidity versus alkalinity
added and (b) settled water turbidity and pH versus alkalinity added. (Adapted from Tseng, T. et al., J. Am. Water Works Assoc., 92(6), 48,
June 2000.)
5.0 pH < 4.5, respectively. Black noted also that when either
• pH was of filtrate used alum or a ferric salt is added to water hydrolysis occurs. The
4.8
for color measurement hydrolysis products were more effective than trivalent ions in
4.6 • TDS = 30 mg/L
pH • HCO = 6.1 mg/L reducing the zeta potential of particles (Black, 1960; Black
–
4.4 3 and Willems, 1961, p. 597). At the same time, Packham
(1962) recognized the role of aluminum hydroxide precipitate,
4.2
that is, that clay particles are ‘‘enmeshed’’ in the ‘‘mass of
4.0 flocculating aluminum hydroxide’’ (later called ‘‘sweep floc’’).
0 100 200 300 400 500
Alum dosage (mg Al (SO ) ·18H O/L) 9.5.3.2 Sequential Hydrolysis Reactions
2
4 3
2
Aluminum or ferric iron salts react with water forming a wide
FIGURE 9.10 pH as affected by alum dosage. (Adapted from
array of complexes or precipitate, depending on pH. To illus-
Black, A.P. and Willems, D.G., J. Am. Water Works Assoc., 53(5),
trate, a postulated sequence of hydrolysis reactions for Al ,
3þ
593, May 1961.)
that is, involving the ‘‘splitting’’ of the water molecule is (Stumm
and Morgan, 1962; Amirtharajah and Mills, 1982, p. 210),
experiments over a 12 month period, the raw water varied
7.0 pH 7.7, 27 alkalinity 30 mg CaCO 3 =L; with Al(H 2 O) 6 3þ þ H 2 O ! Al(H 2 O) (OH) 2þ þ H 3 O þ (Al:1)
5
alum dose 26 mg Al 2 (SO 4 ) 3 14H 2 O=L, the pH just after the
Al(H 2 O) 5 (OH) 2þ þ H 2 O ! Al(H 2 O) 4 (OH) þ H 3 O þ
þ
2
rapid-mix unit was depressed to the range 5.8 pH 6.3
(Hendricks et al., 2000, p. 16). (Al:2)
Al(H 2 O) 4 (OH) 2 þ H 2 O ! Al(H 2 O) 3 (OH) 3 (s) þ H 3 O þ
þ
9.5.3 REACTIONS BETWEEN ALUM=FERRIC IRON (Al:3)
AND WATER
Al(H 2 O) 3 (OH) 3 (s) þ H 2 O ! Al(H 2 O) 2 (OH) 4 þ H 3 O þ
When alum or ferric iron is added to water, the reaction
(Al:4)
product is a complex with six water ligands (i.e., the six
waters each share a coordinated bond with the central metal Al(H 2 O) 2 (OH) 4 þ H 2 O ! Al(H 2 O)(OH) 5 2 þ H 3 O þ
ion). The formula, Al(H 2 O) 6 , was postulated from empir-
3þ
(Al:5)
ical and theoretical considerations and later, in 1972, con-
firmed by ‘‘nuclear-magnetic-resonance’’ spectra (Nordstrom Al(H 2 O)(OH) 5 2 þ H 2 O ! Al(OH) 6 3 þ H 3 O þ (Al:6)
and May, 1996, p. 45).
The foregoing are ‘‘proton-transfer’’ reactions and are essen-
9.5.3.1 Beginning tially instantaneous, limited in rate only by mixing and diffu-
The idea that ferric iron or alum occur as free trivalent ion sion (Nordstrom and May, 1996, p. 44). The hydration waters
began to be questioned by about 1960 when A.P. Black (1960) are ‘‘ligands’’ (see glossary). A similar reaction sequence
recognized that they may be present only at pH < 3.0 and occurs if Fe 3þ is added to water.