Page 247 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 247
202 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
Electrostatic repulsion
Electrostatic repulsion
Negative potential Net potential Negative potential Net potential Negative potential Electrostatic repulsion
Net potential
Energy barrier
Energy barrier Energy barrier
Distance from colloid surface Distance from colloid surface Distance from colloid surface
Energy trap Energy trap Energy trap
Positive potential van der Waal’s Positive potential van der Waal’s Positive potential van der Waal’s
attraction
attraction
attraction
(a) (b) (c)
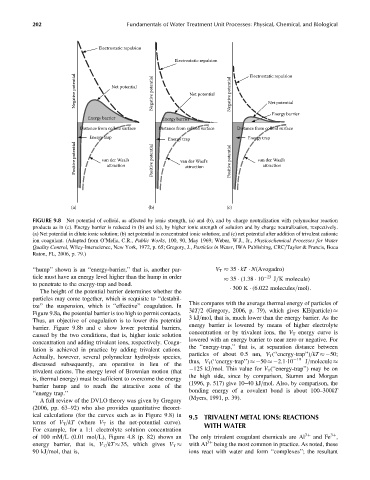
FIGURE 9.8 Net potential of colloid, as affected by ionic strength, (a) and (b), and by charge neutralization with polynuclear reaction
products as in (c). Energy barrier is reduced in (b) and (c), by higher ionic strength of solution and by charge neutralization, respectively.
(a) Net potential in dilute ionic solution; (b) net potential in concentrated ionic solution; and (c) net potential after addition of trivalent cationic
ion coagulant. (Adapted from O’Melia, C.R., Public Works, 100, 90, May 1969; Weber, W.J., Jr., Physicochemical Processes for Water
Quality Control, Wiley-Interscience, New York, 1972, p. 65; Gregory, J., Particles in Water, IWA Publishing, CRC=Taylor & Francis, Boca
Raton, FL, 2006, p. 79.)
‘‘hump’’ shown is an ‘‘energy-barrier,’’ that is, another par- V T 35 kT N(Avogadro)
ticle must have an energy level higher than the hump in order 35 (1:38 10 23 J=K molecule)
to penetrate to the energy-trap and bond.
300 K (6:022 molecules=mol):
The height of the potential barrier determines whether the
particles may come together, which is requisite to ‘‘destabil-
This compares with the average thermal energy of particles of
ize’’ the suspension, which is ‘‘effective’’ coagulation. In
Figure 9.8a, the potential barrier is too high to permit contacts. 3kT=2 (Gregory, 2006, p. 79), which gives KE(particle)
3kJ=mol, that is, much lower than the energy barrier. As the
Thus, an objective of coagulation is to lower this potential
energy barrier is lowered by means of higher electrolyte
barrier. Figure 9.8b and c show lower potential barriers,
concentration or by trivalent ions, the V T energy curve is
caused by the two conditions, that is, higher ionic solution
lowered with an energy barrier to near zero or negative. For
concentration and adding trivalent ions, respectively. Coagu-
the ‘‘energy-trap,’’ that is, at separation distance between
lation is achieved in practice by adding trivalent cations.
particles of about 0.5 nm, V T (‘‘energy-trap’’)=kT 50;
Actually, however, several polynuclear hydrolysis species, 19
thus, V T (‘‘energy-trap’’) 50 2.1 10
discussed subsequently, are operative in lieu of the J=molecule
125 kJ=mol. This value for V T (‘‘energy-trap’’) may be on
trivalent cations. The energy level of Brownian motion (that
the high side, since by comparison, Stumm and Morgan
is, thermal energy) must be sufficient to overcome the energy
(1996, p. 517) give 10–40 kJ=mol. Also, by comparison, the
barrier hump and to reach the attractive zone of the
bonding energy of a covalent bond is about 100–300kT
‘‘energy trap.’’
(Myers, 1991, p. 39).
A full review of the DVLO theory was given by Gregory
(2006, pp. 63–92) who also provides quantitative theoret-
ical calculations (for the curves such as in Figure 9.8) in 9.5 TRIVALENT METAL IONS: REACTIONS
terms of V T =kT (where V T is the net-potential curve). WITH WATER
For example, for a 1:1 electrolyte solution concentration
of 100 mM=L (0.01 mol=L),Figure4.8(p.82) showsan The only trivalent coagulant chemicals are Al 3þ and Fe ,
3þ
with Al 3þ being the most common in practice. As noted, these
energy barrier, that is, V T =kT 35, which gives V T
90 kJ=mol, that is, ions react with water and form ‘‘complexes’’; the resultant