Page 245 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 245
200 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
with three synthetic organics (trichloroethylene, toluene, interface, which may be either negative or positive (usually its
naphthalene), removals were only 5% for each by conven- negative). Five possible causes of the charge at a solid surface
tional filtration (Carlson et al., 1993). In a review of alum include: differential ion solubility; ionization of surface groups;
coagulation of synthetic organics percent removals were: isomorphous ion substitution; specific ion adsorption; aniso-
DDT, 84–95; dieldrin, 10–55; endrin, 0–35; aldrin, 10; tox- tropic crystals (Myers, 1991, p. 71). In each case, a charged
aphene, 0; parathion, 0; malathion, 0; 2,4-D herbicide, 0; chemical group is involved. To illustrate, assume that the
rotenone insecticide, 0 (O’Melia et al., 1979, p. 598). In surface group is COOH. In solution, dissociation occurs,
general, removal of synthetic organic carbon (SOCs) by that is, COOH ! COO þ H . Therefore, the COO group
þ
coagulation is low or uncertain in effectiveness. that remains gives the surface a negative charge but the
system retains overall neutrality, that is, with H going into
þ
the solution.
9.4 DOUBLE LAYER THEORY
The negative surface charge of a colloidal particle (along 9.4.1.3 Gouy–Chapman Model
with small size) causes its special properties, that is, mutual
The Gouy–Chapman model described a diffuse cloud of
repulsion of particles and lack of settling. From this, the
mobile positive point charges surrounding a negatively
‘‘double layer’’ theory has evolved, which is reviewed in
charged colloidal particle. The model, developed by
this section.
G. Gouy in 1910 and D.L. Chapman in 1913, permitted
calculation of distributions of both counterions and electric
9.4.1 DOUBLE LAYER DESCRIPTION potential (O’Melia, 1969). The weakness of the theory when
proposed was that it did not recognize that the point charges
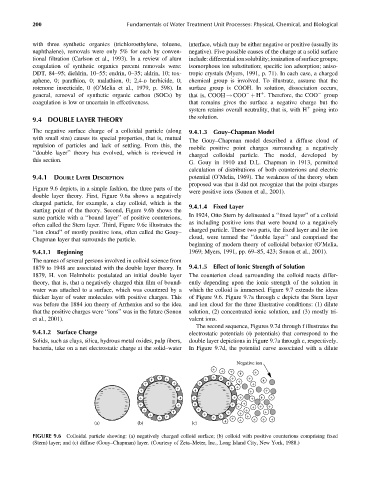
Figure 9.6 depicts, in a simple fashion, the three parts of the
were positive ions (Sonon et al., 2001).
double layer theory. First, Figure 9.6a shows a negatively
charged particle, for example, a clay colloid, which is the 9.4.1.4 Fixed Layer
starting point of the theory. Second, Figure 9.6b shows the
In 1924, Otto Stern by delineated a ‘‘fixed layer’’ of a colloid
same particle with a ‘‘bound layer’’ of positive counterions,
as including positive ions that were bound to a negatively
often called the Stern layer. Third, Figure 9.6c illustrates the
charged particle. These two parts, the fixed layer and the ion
‘‘ion cloud’’ of mostly positive ions, often called the Gouy–
cloud, were termed the ‘‘double layer’’ and comprised the
Chapman layer that surrounds the particle.
beginning of modern theory of colloidal behavior (O’Melia,
9.4.1.1 Beginning 1969; Myers, 1991, pp. 69–85, 423; Sonon et al., 2001).
The names of several persons involved in colloid science from
1879 to 1948 are associated with the double layer theory. In 9.4.1.5 Effect of Ionic Strength of Solution
1879, H. von Helmholtz postulated an initial double layer The counterion cloud surrounding the colloid reacts differ-
theory, that is, that a negatively charged thin film of bound- ently depending upon the ionic strength of the solution in
water was attached to a surface, which was countered by a which the colloid is immersed. Figure 9.7 extends the ideas
thicker layer of water molecules with positive charges. This of Figure 9.6. Figure 9.7a through c depicts the Stern layer
was before the 1884 ion theory of Arrhenius and so the idea and ion cloud for the three illustrative conditions: (1) dilute
that the positive charges were ‘‘ions’’ was in the future (Sonon solution, (2) concentrated ionic solution, and (3) mostly tri-
et al., 2001). valent ions.
The second sequence, Figures 9.7d through f illustrates the
9.4.1.2 Surface Charge electrostatic potentials (c potentials) that correspond to the
Solids, such as clays, silica, hydrous metal oxides, pulp fibers, double layer depictions in Figure 9.7a through c, respectively.
bacteria, take on a net electrostatic charge at the solid–water In Figure 9.7d, the potential curve associated with a dilute
Negative ion
+ + + + +
+ + + + + + + + + +
+ – – – – – – + + – – – – + +
– – – – – – – + + – – – – – + +
–
– – – + – + +
–
– – – – – – – – – – – – – –
– – – – + – – – + + – – – + + + + +
– – – – – – – + – – – +
– –
–
–
– – + – – – + – +
– – – – – – – + + + +
– – – + – – + – – +
– – – – – + – – + + +
–
– – + – + – – +
– – – – + – + +
– – – – + + + + + +
(a) (b) (c) +
FIGURE 9.6 Colloidal particle showing: (a) negatively charged colloid surface; (b) colloid with positive counterions comprising fixed
(Stern) layer; and (c) diffuse (Gouy–Chapman) layer. (Courtesy of Zeta–Meter, Inc., Long Island City, New York, 1988.)