Page 256 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 256
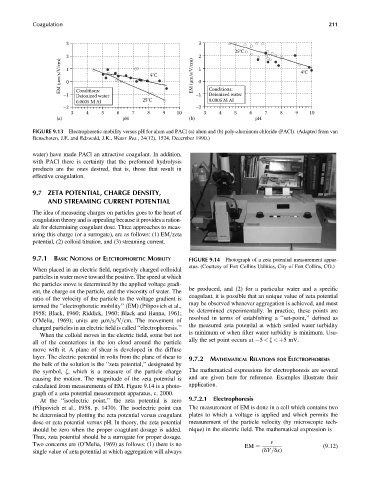
Coagulation 211
3 3
25°C
2 2
EM (μm/s/V/cm) 1 0 4°C EM (μm/s/V/cm) 1 0 4°C
Deionized water
–1 Conditions: –1 Conditions:
Deionized water
0.0005 M Al 25°C 0.0005 M Al
–2 –2
3 4 5 6 7 8 9 10 3 4 5 6 7 8 9 10
(a) pH (b) pH
FIGURE 9.13 Electrophoretic mobility versus pH for alum and PACl (a) alum and (b) poly-aluminum chloride (PACl). (Adapted from van
Benschoten, J.E. and Edzwald, J.K., Water Res., 24(12), 1524, December 1990.)
water) have made PACl an attractive coagulant. In addition,
with PACl there is certainty that the preformed hydrolysis
products are the ones desired, that is, those that result in
effective coagulation.
9.7 ZETA POTENTIAL, CHARGE DENSITY,
AND STREAMING CURRENT POTENTIAL
The idea of measuring charges on particles goes to the heart of
coagulation theory and is appealing because it provides a ration-
ale for determining coagulant dose. Three approaches to meas-
uring this charge (or a surrogate), are as follows: (1) EM=zeta
potential, (2) colloid titration, and (3) streaming current.
9.7.1 BASIC NOTIONS OF ELECTROPHORETIC MOBILITY FIGURE 9.14 Photograph of a zeta potential measurement appar-
atus. (Courtesy of Fort Collins Utilities, City of Fort Collins, CO.)
When placed in an electric field, negatively charged colloidal
particles in water move toward the positive. The speed at which
the particles move is determined by the applied voltage gradi-
be produced, and (2) for a particular water and a specific
ent, the charge on the particle, and the viscosity of water. The
coagulant, it is possible that an unique value of zeta potential
ratio of the velocity of the particle to the voltage gradient is
may be observed whenever aggregation is achieved, and must
termed the ‘‘electrophoretic mobility’’ (EM) (Pilipovich et al.,
be determined experimentally. In practice, these points are
1958; Black, 1960; Riddick, 1960; Black and Hanna, 1961;
resolved in terms of establishing a ‘‘set-point,’’ defined as
O’Melia, 1969); units are mm=s=V=cm. The movement of
charged particles in an electric field is called ‘‘electrophoresis.’’ the measured zeta potential at which settled water turbidity
is minimum or when filter water turbidity is minimum. Usu-
When the colloid moves in the electric field, some but not
ally the set point occurs at 5 < z < þ5 mV.
all of the counterions in the ion cloud around the particle
move with it. A plane of shear is developed in the diffuse
layer. The electric potential in volts from the plane of shear to 9.7.2 MATHEMATICAL RELATIONS FOR ELECTROPHORESIS
the bulk of the solution is the ‘‘zeta potential,’’ designated by
the symbol, z, which is a measure of the particle charge The mathematical expressions for electrophoresis are several
causing the motion. The magnitude of the zeta potential is and are given here for reference. Examples illustrate their
calculated from measurements of EM. Figure 9.14 is a photo- application.
graph of a zeta potential measurement apparatus, c. 2000.
At the ‘‘isoelectric point,’’ the zeta potential is zero 9.7.2.1 Electrophoresis
(Pilipovich et al., 1958, p. 1470). The isoelectric point can The measurement of EM is done in a cell which contains two
be determined by plotting the zeta potential versus coagulant plates to which a voltage is applied and which permits the
dose or zeta potential versus pH. In theory, the zeta potential measurement of the particle velocity (by microscopic tech-
should be zero when the proper coagulant dosage is added. nique) in the electric field. The mathematical expression is
Thus, zeta potential should be a surrogate for proper dosage.
v
Two concerns are (O’Melia, 1969) as follows: (1) there is no (9:12)
EM ¼
single value of zeta potential at which aggregation will always (dV=dx)