Page 53 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 53
8 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
1.3.6 FUTURE OF TREATMENT kind of separation. Then to make any process feasible from an
engineering point of view, there must be a compromise
There is little doubt that technologies will continue to evolve,
between energy cost and the speed of the process (the more
particularly if the market exists for improved applications of
irreversible the process, the higher is its velocity, but the higher
unit processes. Looking at the unit processes, some 15 are
the energy cost). The second law places an inherent limit on
listed in Table 1.1; they were identified based upon funda-
what may be expected.
mental principles. Of the 15 identified, and looking at the
underlying principles of each, the question would be as
follows: Could principles not yet used be applied for separat- 1.4 TREATMENT TRAINS
ing contaminants from water? While any predictions are
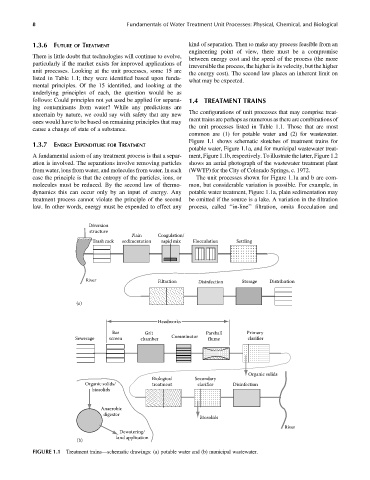
uncertain by nature, we could say with safety that any new The configurations of unit processes that may comprise treat-
ment trains are perhaps as numerous as there are combinations of
ones would have to be based on remaining principles that may
the unit processes listed in Table 1.1. Those that are most
cause a change of state of a substance.
common are (1) for potable water and (2) for wastewater.
Figure 1.1 shows schematic sketches of treatment trains for
1.3.7 ENERGY EXPENDITURE FOR TREATMENT
potable water, Figure 1.1a, and for municipal wastewater treat-
A fundamental axiom of any treatment process is that a separ- ment, Figure1.1b, respectively. Toillustratethe latter, Figure1.2
ation is involved. The separations involve removing particles shows an aerial photograph of the wastewater treatment plant
from water, ions from water, and molecules from water. In each (WWTP) for the City of Colorado Springs, c. 1972.
case the principle is that the entropy of the particles, ions, or The unit processes shown for Figure 1.1a and b are com-
molecules must be reduced. By the second law of thermo- mon, but considerable variation is possible. For example, in
dynamics this can occur only by an input of energy. Any potable water treatment, Figure 1.1a, plain sedimentation may
treatment process cannot violate the principle of the second be omitted if the source is a lake. A variation in the filtration
law. In other words, energy must be expended to effect any process, called ‘‘in-line’’ filtration, omits flocculation and
Diversion
structure
Plain Coagulation/
Trash rack sedimentation rapid mix Flocculation Settling
River Filtration Disinfection Storage Distribution
(a)
Headworks
Bar Grit Parshall Primary
Sewerage screen chamber Comminutor flume clarifier
Organic solids
Biological Secondary
Organic solids/ treatment clarifier Disinfection
biosolids
Anaerobic
digester
Biosolids
River
Dewatering/
land application
(b)
FIGURE 1.1 Treatment trains—schematic drawings: (a) potable water and (b) municipal wastewater.