Page 546 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 546
Adsorption 501
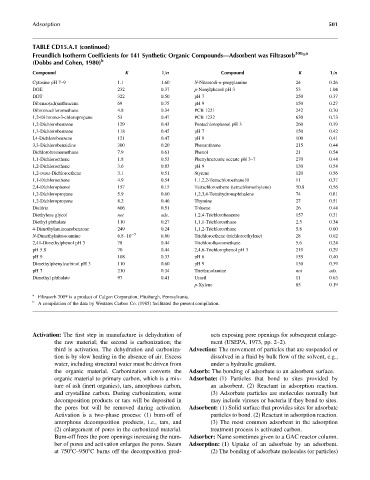
TABLE CD15.A.1 (continued)
t
Freundlich Isotherm Coefficients for 141 Synthetic Organic Compounds—Adsorbent was Filtrasorb 300 a
(Dobbs and Cohen, 1980) b
Compound K 1=n Compound K 1=n
Cytosine pH 7–9 1.1 1.60 N-Nitrosodi-n-propylamine 24 0.26
DOE 232 0.37 p-Nonylphenol pH 3 53 1.04
DDT 322 0.50 pH 7 250 0.37
Dibenzo(a,h)anthracene 69 0.75 pH 9 150 0.27
Dibromochloromethane 4.8 0.34 PCB 1221 242 0.70
1,2-Di bromo-3-chIoropropane 53 0.47 PCB 1232 630 0.73
1,2-Dichlorobenzene 129 0.43 Pentachlorophenol pH 3 260 0.39
1,3-Dichlorobenzene 118 0.45 pH 7 150 0.42
I,4-Dichlorobenzene 121 0.47 pH 9 100 0.41
3,3-Dichlorobenzidine 300 0.20 Phenanthrene 215 0.44
Dichlorobromomethane 7.9 0.61 Phenol 21 0.54
1,1-Dichloroethane 1.8 0.53 Phenylmercuric acetate pH 3–7 270 0.44
1,2-Dichloroethane 3.6 0.83 pH 9 130 0.54
1,2-trans-Dichloroethene 3.1 0.51 Styrene 120 0.56
1,1-Dichloroethene 4.9 0.54 1.1,2,2-Tetrachloroethane10 11 0.37
2,4-Dichlorophenol 157 0.15 Tetrachloroethene (tetrachloroethylene) 50.8 0.56
1,2-Dichloropropane 5.9 0.60 1,2,3,4-Tetrahydronaphthalene 74 0.81
1,2-Dichloropropene 8.2 0.46 Thymine 27 0.51
Dieldrin 606 0.51 Toluene 26 0.44
Diethylene glycol not ads. 1.2.4-Trichlorobenzene 157 0.31
Diethyl phthalate 110 0.27 1,1,1-Trichloroethane 2.5 0.34
4-Dimethylaminoazobenzene 249 0.24 1,1,2-Trichloroethane 5.8 0.60
5
N-Dimethylnitrosoamine 6.8 10 6.60 Trichloroethene (trichloroethylene) 28 0.62
2,41-Dimethylphenol pH 3 78 0.44 Trichlorofluoromethane 5.6 0.24
pH 5.8 70 0.44 2,4,6-Trichlorophenol pH 3 219 0.29
pH 9 108 0.33 pH 6 155 0.40
Dimethylphenylcarbinol pH 3 110 0.60 pH 9 130 0.39
pH 7 210 0.34 Triethanolamine not ads.
Dimethyl phthalate 97 0.41 Uracil 11 0.63
p-Xylene 85 0.19
a
Filtrasorb 300t is a product of Calgon Corporation, Pittsburgh, Pennsylvania.
b
A compilation of the data by Westates Carbon Co. (1985) facilitated the present compilation.
Activation: The first step in manufacture is dehydration of ucts exposing pore openings for subsequent enlarge-
the raw material; the second is carbonization; the ment (USEPA, 1973, pp. 2–2).
third is activation. The dehydration and carboniza- Advection: The movement of particles that are suspended or
tion is by slow heating in the absence of air. Excess dissolved in a fluid by bulk flow of the solvent, e.g.,
water, including structural water must be driven from under a hydraulic gradient.
the organic material. Carbonization converts the Adsorb: The bonding of adsorbate to an adsorbent surface.
organic material to primary carbon, which is a mix- Adsorbate: (1) Particles that bond to sites provided by
ture of ash (inert organics), tars, amorphous carbon, an adsorbent. (2) Reactant in adsorption reaction.
and crystalline carbon. During carbonization, some (3) Adsorbate particles are molecules normally but
decomposition products or tars will be deposited in may include viruses or bacteria if they bond to sites.
the pores but will be removed during activation. Adsorbent: (1) Solid surface that provides sites for adsorbate
Activation is a two-phase process: (1) burn-off of particles to bond. (2) Reactant in adsorption reaction.
amorphous decomposition products, i.e., tars, and (3) The most common adsorbent in the adsorption
(2) enlargement of pores in the carbonized material. treatment process is activated carbon.
Burn-off frees the pore openings increasing the num- Adsorber: Name sometimes given to a GAC reactor column.
ber of pores and activation enlarges the pores. Steam Adsorption: (1) Uptake of an adsorbate by an adsorbent.
at 7508C–9508C burns off the decomposition prod- (2) The bonding of adsorbate molecules (or particles)