Page 562 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 562
Ion-Exchange 517
—
HC CH 2
—
H Vinyl group
H C H HC CH 2 — CH — CH — CH — CH — CH — CH
—
—
C C 2 2 2
C C
—
—
H C HC CH 2
H
(a) H (b) (c) (d)
Styrene
— CH — CH —
— CH — CH 2 2
— CH — CH — CH — CH — DVB
2
2
DVB
SO H — CH—CH — CH — CH —
2
2
3
Sulfonic acid
— CH — CH — CH — CH — group with H +
2
2
counter ion
(e) Styrene (f) SO H
3
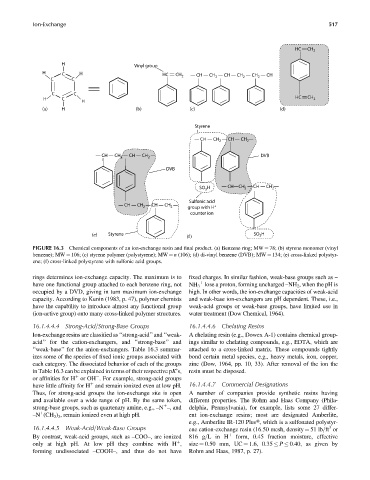
FIGURE 16.3 Chemical components of an ion-exchange resin and final product. (a) Benzene ring; MW ¼ 78; (b) styrene monomer (vinyl
benzene); MW ¼ 106; (c) styrene polymer (polystyrene); MW ¼ n (106); (d) di-vinyl benzene (DVB); MW ¼ 134; (e) cross-linked polystyr-
ene; (f) cross-linked polystyrene with sulfonic acid groups.
rings determines ion-exchange capacity. The maximum is to fixed charges. In similar fashion, weak-base groups such as –
have one functional group attached to each benzene ring, not NH 3 lose a proton, forming uncharged –NH 2 , when the pH is
þ
occupied by a DVD, giving in turn maximum ion-exchange high. In other words, the ion-exchange capacities of weak-acid
capacity. According to Kunin (1983, p. 47), polymer chemists and weak-base ion-exchangers are pH dependent. These, i.e.,
have the capability to introduce almost any functional group weak-acid groups or weak-base groups, have limited use in
(ion-active group) onto many cross-linked polymer structures. water treatment (Dow Chemical, 1964).
16.1.4.4.4 Strong-Acid=Strong-Base Groups 16.1.4.4.6 Chelating Resins
Ion-exchange resins are classified as ‘‘strong-acid’’ and ‘‘weak- A chelating resin (e.g., Dowex A-1) contains chemical group-
acid’’ for the cation-exchangers, and ‘‘strong-base’’ and ings similar to chelating compounds, e.g., EDTA, which are
‘‘weak-base’’ for the anion-exchangers. Table 16.3 summar- attached to a cross-linked matrix. These compounds tightly
izes some of the species of fixed ionic groups associated with bond certain metal species, e.g., heavy metals, iron, copper,
each category. The dissociated behavior of each of the groups zinc (Dow, 1964, pp. 10, 33). After removal of the ion the
in Table 16.3 can be explained in terms of their respective pK’s, resin must be disposed.
or affinities for H or OH . For example, strong-acid groups
þ
have little affinity for H and remain ionized even at low pH. 16.1.4.4.7 Commercial Designations
þ
Thus, for strong-acid groups the ion-exchange site is open A number of companies provide synthetic resins having
and available over a wide range of pH. By the same token, different properties. The Rohm and Haas Company (Phila-
strong-base groups, such as quartenary amine, e.g., –N –, and delphia, Pennsylvania), for example, lists some 27 differ-
þ
–N (CH 3 ) 3 , remain ionized even at high pH. ent ion-exchange resins; most are designated Amberlite,
þ
e.g., Amberlite IR-120 Plust, which is a sulfonated polystyr-
16.1.4.4.5 Weak-Acid=Weak-Base Groups ene cation-exchange resin (16.50 mesh, density ¼ 51 lb=ft or
3
By contrast, weak-acid groups, such as –COO–, are ionized 816 g=LinH þ form, 0.45 fraction moisture, effective
only at high pH. At low pH they combine with H , size ¼ 0.50 mm, UC ¼ 1.6, 0.35 P 0.40, as given by
þ
forming undissociated –COOH–, and thus do not have Rohm and Haas, 1987, p. 27).