Page 565 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 565
520 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
Typically, the granules for packed-beds are 28–48 mesh the ion is being removed, which may be seen in terms of an
(0.3–0.6 mm). The site charges are related to pH as follows isotherm. For a given influent concentration of counterion,
(Carlson and Thomson, 2001): e.g., ‘‘A ,’’ C o (A ), to a packed-bed, the quantity adsorbed is
þ
þ
X*(‘‘A ’’).
þ
þ
At pH < pH zpc , the sites are charged as OH 2
16.2.1.1 Expressions of Capacity
At pH ¼ pH ZPC , the sites are charged as OH
At pH > pH ZPC , the sites are charged as O Helfferich (1962) lists seven expressions of ion-exchanger
For reference, pH ZPC ¼ 8.2–9.1, where pH ZPC is the pH capacity and mentions that confusion often is the result. The
at zero-point charge ‘‘mass capacity’’ is recommended, and is defined as ‘‘milliequi-
valents of exchange capacity per gram of dry resin,’’ or meq=g
dry resin. A holdover expression from the early days of soft-
Regarding the background on the development of practice, 3
ening practice is ‘‘kilograins as CaCO 3 =ft .’’ Conversions
Savinelli and Black (1958, p. 34) reviewed the use of acti-
between various units of capacity are given in Appendix 16.A.
vated alumina for fluoride removal in a full-scale plant con-
3
3
structed in 1952 at Bartlett, Texas using a 14.15 m (500 ft )
bed. They cited C.S. Boruff as the person who initiated the 16.2.1.2 Upper Limit of Capacity
development of activated alumina, who in 1934 described The upper limit of ion-exchanger ‘‘capacity’’ is the number of
regeneration with NaOH, followed by neutralization with sites per unit mass of dry media, designated as X M , expressed
HCl. A 1936 patent by H.V. Churchill mentioned specifically usually in milliequivalents of sites per gram of dry media. For
the use of activated alumina (as contrasted with aluminum a zeolite, X M must be determined empirically. For resin, X M
oxide). The alumina was ‘‘activated’’ by heating it to 4008C– may be calculated as illustrated in Example 16.1.
5008C in the presence of alkali metal ions; the heating was the
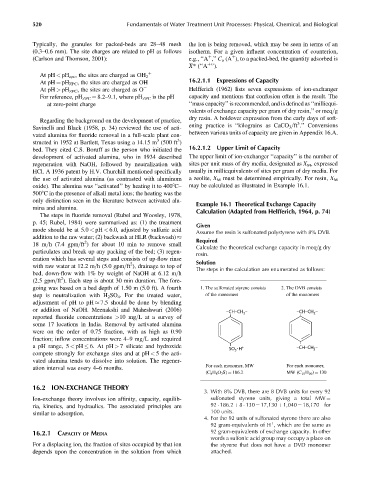
only distinction seen in the literature between activated alu- Example 16.1 Theoretical Exchange Capacity
mina and alumina. Calculation (Adapted from Helfferich, 1964, p. 74)
The steps in fluoride removal (Rubel and Woosley, 1978,
p. 45; Rubel, 1984) were summarized as: (1) the treatment
Given
mode should be at 5.0 < pH < 6.0, adjusted by sulfuric acid
Assume the resin is sulfonated polystyrene with 8% DVB.
addition to the raw water; (2) backwash at HLR (backwash) Required
2
18 m=h (7.4 gpm=ft ) for about 10 min to remove small
Calculate the theoretical exchange capacity in meq=g dry
particulates and break up any packing of the bed; (3) regen-
resin.
eration which has several steps and consists of up-flow rinse
2 Solution
with raw water at 12.2 m=h (5.0 gpm=ft ), drainage to top of
The steps in the calculation are enumerated as follows:
bed, down-flow with 1% by weight of NaOH at 6.12 m=h
2
(2.5 gpm=ft ). Each step is about 30 min duration. The fore-
going was based on a bed depth of 1.50 m (5.0 ft). A fourth 1. The sulfonated styrene consists 2. The DVB consists
step is neutralization with H 2 SO 4 . For the treated water, of the monomers of the monomers
adjustment of pH to pH 7.5 should be done by blending
or addition of NaOH. Meenakshi and Maheshwari (2006) –CH–CH – –CH–CH –
2
2
reported fluoride concentrations >10 mg=L at a survey of
some 17 locations in India. Removal by activated alumina
were on the order of 0.75 fraction, with as high as 0.90
fraction; inflow concentrations were 4–9mg=L and required
a pH range, 5 < pH 6. At pH > 7 silicate and hydroxide + –CH–CH –
SO 3 • H 2
compete strongly for exchange sites and at pH < 5 the acti-
vated alumina tends to dissolve into solution. The regener-
For each monomer, MW For each monomer,
ation interval was every 4–6 months.
(C 8 H 8 O 3 S) ¼ 186.2 MW (C 10 H 10 ) ¼ 130
16.2 ION-EXCHANGE THEORY
3. With 8% DVB, there are 8 DVB units for every 92
Ion-exchange theory involves ion affinity, capacity, equilib- sulfonated styrene units, giving a total MW ¼
ria, kinetics, and hydraulics. The associated principles are 92 186.2 þ 8 130 ¼ 17,130 þ 1,040 ¼ 18,170 for
100 units.
similar to adsorption.
4. For the 92 units of sulfonated styrene there are also
92 gram-equivalents of H , which are the same as
þ
92 gram-equivalents of exchange capacity. In other
16.2.1 CAPACITY OF MEDIA
words a sulfonic acid group may occupy a place on
For a displacing ion, the fraction of sites occupied by that ion the styrene that does not have a DVD monomer
depends upon the concentration in the solution from which attached.