Page 568 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 568
Ion-Exchange 523
Fick’s second law is a materials balance relationship. 16.3 DESIGN
Assume the ion-exchanger particle has spherical geometry
Design involves not only selection of an ion-exchanger, but
and D A is constant, then combining (16.8) and (16.9) gives
sizing of the support components with suitable materials.
Much of this information is available in manufacturers’ design
qC A qC A 2qC A manuals in hard copy (e.g., Dow Chemical, 1964; Rohm and
¼ D A þ (16:10)
qt qr 2 rqr Haas, 1987) or in Adobe Acrobatt format for a Web site (e.g.,
http:==www.purolite.com=Re1Id=33637=ISvars=default/Home.
htm, 1999).
16.2.3.2.2 Film Diffusion Control
The flux across the film around the ion-exchanger particle is
16.3.1 SELECTION OF ION-EXCHANGERS
The particular ion-exchanger used depends upon the purpose
DC A
j A ¼ D A (16:11) (e.g., softening, demineralization, ammonia removal from
d
wastewaters, nitrate removal, boron removal, heavy-metal
removal), properties desired (e.g., high exchange capacity,
in which
minimal swelling, no attrition), and cost. In addition, site
D A is the diffusion coefficient of A in the liquid phase
þ
2
(cm =s) specific factors may be overriding (e.g., availability of natural
zeolites, cost of re-generant chemicals, disposal issues).
DC A is the concentration difference across film, i.e., from
the bulk of solution to the ion-exchanger external sur- 16.3.1.1 Resins
face (mg A =mL)
þ
For most water treatment applications, a polystyrene with DVB
d is the film thickness (cm)
cross-linking, strong-acid cation-exchange resin is recom-
16.2.3.2.3 Diffusion Coefficients mended, having SO 3 as the functional group. For the removal
of anions, as in demineralization, a strong-base anion-exchanger
The diffusion coefficient, D A , of a species A within the ion-
þ
having a quaternary amine functional group is commonly used.
exchanger depends on the size, valence, and chemical nature
The suitability of the resin selected should be confirmed by
of species A , the degree of swelling, mesh width, charge
þ
laboratory tests and, preferably, by pilot plant testing.
density, chemical nature of the solid phase matrix, compos-
The laboratory testing can provide data on fundamental param-
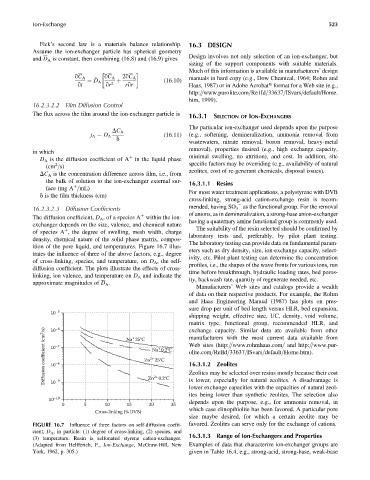
ition of the pore liquid, and temperatures. Figure 16.7 illus-
eters such as dry density, size, ion-exchange capacity, select-
trates the influence of three of the above factors, e.g., degree
ivity, etc. Pilot plant testing can determine the concentration
of cross-linking, species, and temperature, on D A , the self-
profiles, i.e., the shapes of the wave fronts for various ions, run
diffusion coefficient. The plots illustrate the effects of cross-
time before breakthrough, hydraulic loading rates, bed poros-
linking, ion valence, and temperature on D A and indicate the
ity, backwash rate, quantity of regenerate needed, etc.
approximate magnitudes of D A .
Manufacturers’ Web sites and catalogs provide a wealth
of data on their respective products. For example, the Rohm
and Haas Engineering Manual (1987) has plots on pres-
sure drop per unit of bed length versus HLR, bed expansion,
10 –5
shipping weight, effective size, UC, density, void volume,
matrix type, functional group, recommended HLR, and
Diffusion coefficient (cm 2 /s) 10 –7 Zn 25°C Web sites (http:==www.rohmhaas.com= and http:==www.pur-
–6
exchange capacity. Similar data are available from other
10
manufacturers with the most current data available from
+
Na 25°C
+
Na 0.2°C
olite.com=RelId=33637=ISvars=default=Home.htm).
2+
16.3.1.2
Zeolites
–8
10
Zeolites may be selected over resins mostly because their cost
2+
Zn 0.2°C
is lower, especially for natural zeolites. A disadvantage is
–9
10
lower exchange capacities with the capacities of natural zeol-
ites being lower than synthetic zeolites. The selection also
10 –10
0 5 10 15 20 25 depends upon the purpose, e.g., for ammonia removal, in
which case clinopthiolite has been favored. A particular pore
Cross-linking (% DVB)
size maybe desired, for which a certain zeolite may be
FIGURE 16.7 Influence of three factors on self-diffusion coeffi- favored. Zeolites can serve only for the exchange of cations.
cient, D A , in particle: (1) degree of cross-linking, (2) species, and
16.3.1.3 Range of Ion-Exchangers and Properties
(3) temperature. Resin is sulfonated styrene cation-exchanger.
(Adapted from Helfferich, F., Ion-Exchange, McGraw-Hill, New Examples of data that characterize ion-exchanger groups are
York, 1962, p. 305.) given in Table 16.4, e.g., strong-acid, strong-base, weak-base