Page 573 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 573
528 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
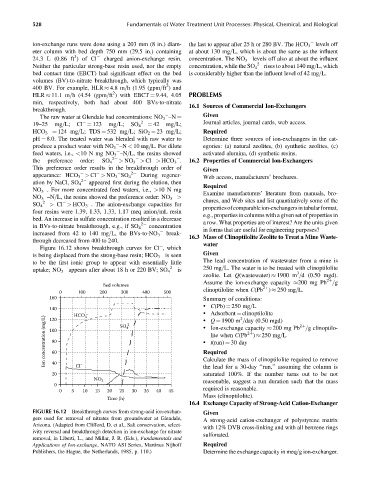
ion-exchange runs were done using a 203 mm (8 in.) diam- the last to appear after 25 h or 280 BV. The HCO 3 levels off
eter column with bed depth 750 mm (29.5 in.) containing at about 130 mg=L, which is about the same as the influent
3
24.3 L (0.86 ft )ofCl charged anion-exchange resin. concentration. The NO 3 levels off also at about the influent
Neither the particular strong-base resin used, nor the empty concentration, while the SO 4 2 rises to about 140 mg=L, which
bed contact time (EBCT) had significant effect on the bed is considerably higher than the influent level of 42 mg=L.
volumes (BV)-to-nitrate breakthrough, which typically was
2
400 BV. For example, HLR 4.8 m=h (1.95 (gpm=ft ) and
2
HLR 11.1 m=h (4.54 (gpm=ft ) with EBCT ¼ 9.44, 4.05 PROBLEMS
min, respectively, both had about 400 BVs-to-nitrate
16.1 Sources of Commercial Ion-Exchangers
breakthrough.
Given
The raw water at Glendale had concentrations: NO 3 –N ¼
19–25 mg=L; Cl ¼ 123 mg=L; SO 4 2 ¼ 42 mg=L; Journal articles, journal cards, web access.
HCO 3 ¼ 124 mg=L; TDS ¼ 532 mg=L; SiO 2 ¼ 23 mg=L; Required
pH ¼ 8.0. The treated water was blended with raw water to Determine three sources of ion-exchangers in the cat-
produce a product water with NO 3 –N < 10 mg=L. For dilute egories: (a) natural zeolites, (b) synthetic zeolites, (c)
feed waters, i.e., <10 N mg NO 3 –N=L, the resins showed activated alumina, (d) synthetic resins.
the preference order: SO 4 2 > NO 3 > Cl > HCO 3 . 16.2 Properties of Commercial Ion-Exchangers
This preference order results in the breakthrough order of Given
2
appearance: HCO 3 > Cl > NO 3 SO 4 During regener- Web access, manufacturers’ brochures.
2
ation by NaCl, SO 4 appeared first during the elution, then
Required
NO 3 . For more concentrated feed waters, i.e., >10 N mg
NO 3 –N=L, the resins showed the preference order: NO 3 > Examine manufacturers’ literature from manuals, bro-
chures, and Web sites and list quantitatively some of the
2
SO 4 > Cl > HCO 3 . The anion-exchange capacities for
propertiesofcomparableion-exchangersintabularformat,
four resins were 1.39, 1.33, 1.33, 1.17 meq anion=mL resin
e.g., properties in columns with a given set of properties in
bed. An increase in sulfate concentration resulted in a decrease
a row. What properties are of interest? Are the units given
2
in BVs-to-nitrate breakthrough, e.g., if SO 4 concentration
in forms that are useful for engineering purposes?
increased from 42 to 140 mg=L, the BVs-to-NO 3 break-
16.3 Mass of Clinoptilolite Zeolite to Treat a Mine Waste-
through decreased from 400 to 240.
water
Figure 16.12 shows breakthrough curves for Cl , which
is being displaced from the strong-base resin; HCO 3 is seen Given
to be the first ionic group to appear with essentially little The lead concentration of wastewater from a mine is
2 is 250 mg=L. The water is to be treated with clinoptilolite
uptake; NO 3 appears after about 18 h or 220 BV; SO 4 3
zeolite. Let Q(wastewater) 1900 m =d (0.50 mgd).
Assume the ion-exchange capacity 200 mg Pb =g
2þ
Bed volumes
clinoptilolite when C(Pb ) 250 mg=L.
2þ
0 100 200 300 400 500
160 Summary of conditions:
. C(Pb) ¼ 250 mg=L
140 HCO 3 – . Adsorbent ¼ clinoptilolite
Ion concentration (mg/L) 100 SO 4 2 Required 2þ 2þ
3
120
. Q ¼ 1900 m =day (0.50 mgd)
. Ion-exchange capacity 200 mg Pb =g clinoptilo-
lite when C(Pb ) 250 mg=L
80
. t(run) ¼ 30 day
60
40
the lead for a 30-day ‘‘run,’’ assuming the column is
saturated 100%. If the number turns out to be not
20 Cl – Calculate the mass of clinoptilolite required to remove
–
NO 3 reasonable, suggest a run duration such that the mass
0
0 5 10 15 20 25 30 35 40 45 required is reasonable.
Mass (clinoptilolite).
Time (h)
16.4 Exchange Capacity of Strong-Acid Cation-Exchanger
FIGURE 16.12 Breakthrough curves from strong-acid ion-exchan- Given
gers used for removal of nitrates from groundwater at Glendale, A strong-acid cation-exchanger of polystyrene matrix
Arizona. (Adapted from Clifford, D. et al., Salt conservation, select-
with 12% DVB cross-linking and with all benzene rings
ivity reversal and breakthrough detection in ion-exchange for nitrate
sulfonated.
removal, in Liberti, L., and Millar, J. R. (Eds.), Fundamentals and
Applications of Ion-exchange, NATO ASI Series, Martinus Nijhoff Required
Publishers, the Hague, the Netherlands, 1985, p. 110.) Determine the exchange capacity in meq=g ion-exchanger.