Page 709 - Fundamentals of Water Treatment Unit Processes : Physical, Chemical, and Biological
P. 709
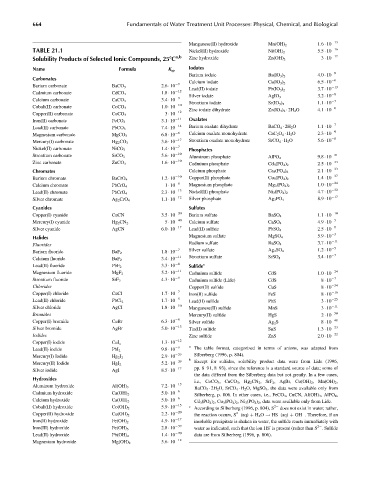
664 Fundamentals of Water Treatment Unit Processes: Physical, Chemical, and Biological
Manganese(II) hydroxide Mn(OH) 2 1.6 10 13
TABLE 21.1 Nickel(II) hydroxide Ni(OH) 2 5.5 10 16
Solubility Products of Selected Ionic Compounds, 258C a,b Zinc hydroxide Zn(OH) 2 3 10 17
Name Formula K sp Iodates
Barium iodate Ba(IO 3 ) 2 4.0 10 9
Carbonates
Calcium iodate Ca(IO 3 ) 2 6.5 10 6
Barium carbonate BaCO 3 2.6 10 9
Lead(II) iodate Pb(IO 3 ) 2 3.7 10 13
Cadmium carbonate CdCO 3 1.8 10 12 Silver iodate 3.2 10 8
Calcium carbonate CaCO 3 3.4 10 9 AgIO 3
Strontium iodate Sr(IO 3 ) 2 1.1 10 7
Cobalt(II) carbonate CoCO 3 1.0 10 10 Zinc iodate dihydrate Zn(IO 3 ) 2 2H 2 O 4.1 10 6
Copper(II) carbonate CuCO 3 3 10 12
Iron(II) carbonate FeCO 3 3.1 10 11 Oxalates
Lead(II) carbonate PbCO 3 7.4 10 14 Barium oxalate dihydrate BaCO 4 2H 2 O 1.1 10 7
Magnesium carbonate MgCO 3 6.8 10 6 Calcium oxalate monohydrate CaC 2 O 4 H 2 O 2.3 10 9
Mercury(I) carbonate Hg 2 CO 3 3.6 10 17 Strontium oxalate monohydrate SrCO 4 H 2 O 5.6 10 8
Nickel(II) carbonate NiCO 3 1.4 10 7 Phosphates
10
Strontium carbonate SrCO 3 5.6 10 Aluminum phosphate AlPO 4 9.8 10 21
Zinc carbonate ZnCO 3 1.6 10 10 Cadmium phosphate Cd 3 (PO 4 ) 2 2.5 10 33
33
Chromates Calcium phosphate Ca 3 (PO 4 ) 2 2.1 10
Barium chromate BaCrO 4 1.2 10 10 Copper(II) phosphate Cu 3 (PO 4 ) 2 1.4 10 37
Calcium chromate PbCrO 4 1 10 8 Magnesium phosphate Mg 3 (PO 4 ) 2 1.0 10 24
Lead(II) chromate PbCrO 4 2.3 10 13 Nickel(II) phosphate Ni 3 (PO 4 ) 2 4.7 10 32
Silver chromate Ag 2 CrO 4 1.1 10 12 Silver phosphate Ag 3 PO 4 8.9 10 17
Cyanides Sulfates
Copper(I) cyanide CuCN 3.5 10 20 Barium sulfate BaSO 4 1.1 10 10
Mercury(I) cyanide Hg 2 CN 2 5 10 40 Calcium sulfate CaSO 4 4.9 10 5
Silver cyanide AgCN 6.0 10 17 Lead(II) sulfate PbSO 4 2.5 10 8
3
5.9 10
Magnesium sulfate
Halides MgSO 4
Radium sulfate 3.7 10 11
Fluorides RaSO 4
5
7 Silver sulfate 1.2 10
Barium fluoride BaF 2 1.8 10 Ag 2 SO 4
Calcium fluoride BaF 2 3.4 10 11 Strontium sulfate SrSO 4 3.4 10 7
Lead(II) fluoride PbF 2 3.3 10 8 Sulfide c
Magnesium fluoride MgF 2 5.2 10 11 Cadmium sulfide CdS 1.0 10 24
Strontium fluoride SrF 2 4.3 10 9 Cadmium sulfide (Lide) CdS 8 10 7
Chlorides Copper(II) sulfide CuS 8 10 34
Copper(I) chloride CuCl 1.7 10 7 Iron(II) sulfide FeS 8 10 16
Lead(II) chloride PbCl 2 1.7 10 5 Lead(II) sulfide PbS 3 10 25
Silver chloride AgCl 1.8 10 10 Manganese(II) sulfide MnS 3 10 11
Bromides Mercury(II) sulfide HgS 2 10 50
Copper(I) bromide CuBr 6.3 10 9 Silver sulfide Ag 2 S 8 10 48
13
Silver bromide AgBr 5.0 10 Tin(II) sulfide SnS 1.3 10 23
Iodides Zinc sulfide ZnS 2.0 10 22
Copper(I) iodide CuI x 1.3 10 12
Lead(II) iodide PbI 2 9.8 10 9 a The table format, categorized in terms of anions, was adapted from
Mercury(I) Iodide Hg 2 I 2 2.9 10 29 Silberberg (1996, p. 804).
b Except for sulfides, solubility product data were from Lide (1996,
Mercury(II) Iodide HgI 2 5.2 10 29
Silver iodide AgI 8.5 10 17 pp. 8–91, 8–93), since the reference is a standard source of data; some of
the data differed from the Silberberg data but not greatly. In a few cases,
Hydroxides
i.e., CoCO 3 , CuCO 3 ,Hg 2 CN 2 , SrF 2 , AgBr, Cu(OH) 2 , Mn(OH) 2 ,
Aluminum hydroxide 7.2 10 15
Al(OH) 3
BaCO 4 2H 2 O, SrCO 4 H 2 O, MgSO 4 , the data were available only from
Cadmium hydroxide 5.0 10 6
Silberberg, p. 806. In other cases, i.e., FeCO 3 , CuCN, Al(OH) 3 , AlPO 4 ,
Ca(OH) 2
Calcium hydroxide Ca(OH) 2 5.0 10 6 Cd 3 (PO 4 ) 2 ,Cu 3 (PO 4 ) 2 ,Ni 3 (PO 4 ) 2 , data were available only from Lide.
15
Cobalt(II) hydroxide Co(OH) 2 5.9 10 c According to Silberberg (1996, p. 804), S 2 does not exist in water; rather,
Copper(II) hydroxide Cu(OH) 2 2.2 10 20 the reaction occurs, S (aq) þ H 2 O ! HS (aq) þ OH . Therefore, if an
2
Iron(II) hydroxide Fe(OH) 2 4.9 10 17 insoluble precipitate is shaken in water, the sulfide reacts immediately with
-
Iron(III) hydroxide Fe(OH) 3 2.8 10 39 water as indicated, such that the ion HS is present (rather than S . Sulfide
2
Lead(II) hydroxide Pb(OH) 2 1.4 10 20 data are from Silberberg (1996, p. 806).
Magnesium hydroxide Mg(OH) 2 5.6 10 12