Page 142 - Gas Purification 5E
P. 142
132 Gas Purification
react with C02 to form carbamates, which are difficult to decompose. Therefore, for these
amines a significant amount of CO, may be present at the bottom of the regenerator, and
enhanced stripping of H2S may be realized. HGh regenerator pressures may also enhance
HzS stripping when dissolved COz is present by forcing the release of additional COz with
its accompanying equilibrium concentration of H2S.
The “common ion” effect has also been used to advantage in both the Sulften and Flex-
sorb SE+ Claus tail gas processes. In both of these processes, acids are added to enhance
amine solution stripping (Dibble, 1985; Ho et al., 1990). Sulften is an MDEA-based process;
while the Flexsorb SE+ process uses a hindered amine. With both processes, Claus plant tail
gas H2S emissions are reduced from 250 to less than 10 ppmv (Tragitt et al., 1986; Ho et al.,
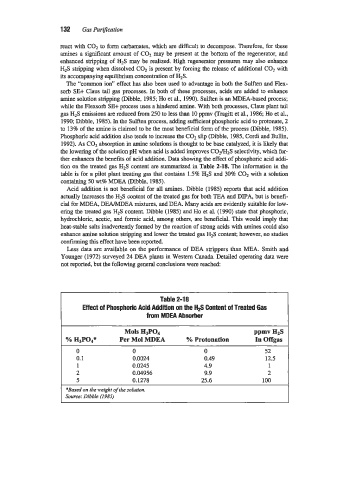
1990; Dibble, 1985). In the Sulften process, adding sufficient phosphoric acid to protonate, 2
to 138 of the amine is claimed to be the most beneficial form of the process (Dibble, 1985).
Phosphoric acid addition also tends to increase the C02 slip (Dibble, 1985, Cordi and Bullin,
1992). As CO, absorption in amine solutions is thought to be base catalyzed, it is likely that
the lowering of the solution pH when acid is added improves C02/HzS selectiwity, which fur-
ther enhances the benefits of acid addition. Data showing the effect of phosphoric acid addi-
tion on the treated gas H2S content are summarized in Table 2-18. The information in the
table is for a pilot plant treating gas that contains 1.5% HzS and 30% C02 with a solution
containing 50 wt8 MDEA (Dibble, 1985).
Acid addition is not beneficial for all amines. Dibble (1985) reports that acid addition
actually increases the H2S content of the treated gas for both TEA and DIP& but is benefi-
cial for MDEA, DEAMDEA mixtures, and DEA. Many acids are evidently suitable for low-
ering the treated gas H2S content. Dibble (1985) and Ho et al. (1990) state that phosphoric,
hydrochloric, acetic, and formic acid, among others, are beneficial. This would imply that
heat-stable salts inadvertently formed by the reaction of strong acids with amines could also
enhance amine solution stripping and lower the treated gas H2S content: however, no studies
confirming this effect have been reported.
Less data are available on the performance of DEA strippers than MEA. Smith and
Younger (1972) surveyed 24 DEA plants in Western Canada. Detailed operating data were
not reported, but the following general conclusions were reach&
Table 2-1 8
EfFect of Phosphohc Acid Addition on the Hfi Content of Treated Gas
from MDEA Absorber
Mols H3P04 ppmvH2s I
I %&Pod* Per Mol MDEA YO Protonation In Offgas
0 0 0 52
0.1 0.0024 0.49 12.5
1 0.0245 4.9 1
2 0.04956 9.9 2
5 0.1278 25.6 100
*Based on the weight of the solution.
Source: Dibble (1985)