Page 206 - Gas Purification 5E
P. 206
192 GasPuriJcation
The specific acid anion can also affect the corrosion rate. Some acids, such as H2S, react
with iron to form a relatively dense, non-porous scale that can significantly reduce continued
corrosion. Other acids, such as oxalic, tend to increase solution corrosivity due to the forma-
tion of soluble iron chelates (Rooney et al., 1996).
An alternative explanation for the high rate of corrosion of iron in contact with C02-con-
taining water is described by de Waard and Lotz (1993). In the proposed mechanism, car-
bonic acid, H2C03, is formed by hydration of dissolved C02. It is postulated that the carbon-
ic acid molecule participates directly in the corrosion reaction by accepting an electron from
the corroding iron to form HC03- and elemental hydrogen in accordance with the following
equation:
H2C03 + e- = H" + HC03- (3-5)
The rate of corrosion by this mechanism would, of course, increase with increased carbon
dioxide partial pressure and be affected by temperature.
Whether the primary mechanism for the corrosion of iron by carbon dioxide and water
involves the acceptance of electrons from hydrogen ions or from molecular carbonic acid,
the overall reaction is the same, i.e.:
Fe + 2C02 + 2H20 = Fez+ + 2HC03- + 2H" (3-6)
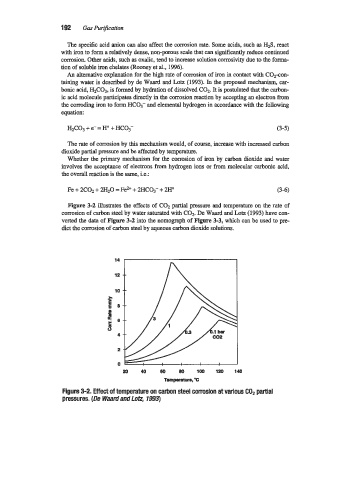
Figure 3-2 illustrates the effects of C02 partial pressure and temperature on the rate of
corrosion of carbon steel by water saturated with COP De Waard and Lotz (1993) have con-
verted the data of Figure 3-2 into the nomograph of Figure 3-3, which can be used to pre-
dict the cxmosion of carbon steel by aqueous carbon dioxide solutions.
14
12
10
-E
ill
6"
4
2
0
20 40 80 80 100 120 140
Tempamturn. F
Figure 3-2. Effect of temperature on carbon steel corrosion at various COP partial
pressures. (De Waard and Lo&, 1993)