Page 207 - Gas Purification 5E
P. 207
Mechanical Design and Operation of Alkanolamine Plants 193
Figure 3-2 shows that the corrosion rate at any given partial pressure increases with tem-
perature at moderate temperatures, but reaches a maximum as the temperature is further
increased. De Waard and Lotz (1993) attribute this effect to the formation of a more protec-
tive film, either FeC03 or Fe304, at higher temperatures. The reduction of comsion rate at
elevated temperatures is accounted for in the nomograph by the inclusion of a scale factor
from 0.1 to 1, which is used as a multiplier to reduce the corrosion rate read directly from the
chart in the higher temperature region.
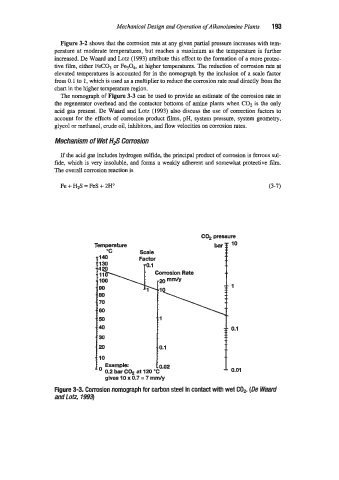
The nomograph of Figure 3-3 can be used to provide an estimate of the corrosion rate in
the regenerator overhead and the contactor bottoms of amine plants when COz is the only
acid gas present. De Waard and Lotz (1993) also discuss the use of correction factors to
account for the effects of corrosion product flms, pH, system pressure, system geometry,
glycol or methanol, crude oil, inhibitors, and flow velocities on umusion rates.
Mechanism of Wet Hfi Corrosion
If the acid gas includes hydrogen sultide, the principal product of corrosion is ferrous sul-
fide, which is vay insoluble, and fom a weakly adherent and somewhat protective film.
The overall corrosion reaction is
Fe + H2S = FeS + 2H" (3-7)
Figure 3-3. Corrosion nomograph for cahon steel in contact with wet COP. (De Waard
and f otz, 1993)