Page 97 - Gas Purification 5E
P. 97
Alkanolamines for Hydrogen Surfide and Carbon Dioxide Removal 87
1 o2
10'
h
g loo
Y
8-
a
lo-'
1 o-2
I
0 5 10 15 20 25 30
-MEA
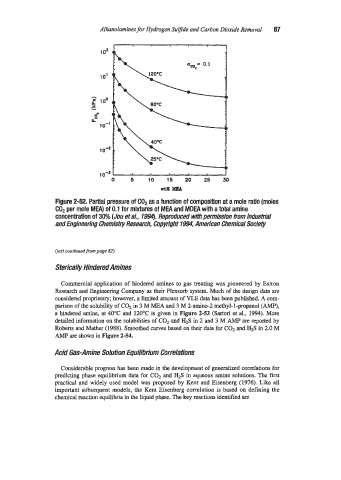
Figure 2-52. Partial pressure of GO2 as a function of composition at a mole ratio (moles
COP per mole MEA) of 0.1 for mixtures of MEA and MDEA with a total amine
concentration of 30% (Jou et ai., 1994). Reproduced with permission from Industrial
and Engineering Chemism Research, Copyright 7994, American Chemical Society
(text continuedfronz page 82)
Sterically Hindered Amines
Commercial application of hindered amines to gas treating was pioneered by Exxon
Research and Engineering Company as their Flexsorb system. Much of the design data are
considered proprietary; however, a limited amount of VLE data has been published. A com-
parison of the solubility of C02 in 3 M MEA and 3 M 2-amino-2 methyl-I-propanol (AMP),
a hindered amine, at 40°C and 120°C is given in Figure 2-53 (Sartori et al., 1994). More
detailed information on the solubilities of COz and H2S in 2 and 3 M AMP are reported by
Roberts and Mather (1988). Smoothed curves based on their data for COz and HzS in 2.0 M
AMP are shown in Figure 2-54.
Acid Gas-Amine Solution Equilibrium Correlations
Considerable progress has been made in the development of generalized correlations for
predicting phase equilibrium data for COz and H2S in aqueous amine solutions. The first
practical and widely used model was proposed by Kent and Eisenberg (1976). Like all
important subsequent models, the Kent Eisenberg correlation is based on defining the
chemical reaction equilibria in the liquid phase. The key reactions identified are