Page 101 - Gas Purification 5E
P. 101
Alkanolamines for Hydrogen Surfide and carbon Dioxide Removal 91
0.0 0.2 0.4 0.6 C.8 1.0
loading (mol col/md amine)
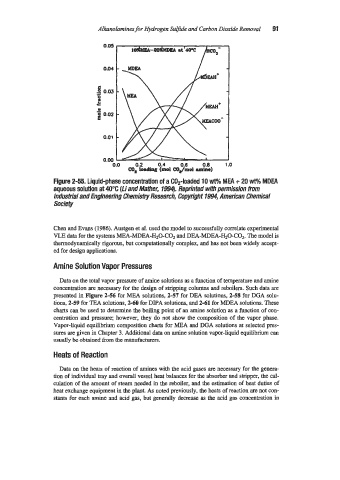
Figure 2-55. Liquid-phase concentration of a Cop-loaded 10 wt% MEA + 20 Wpm MDEA
aqueous solutian at 40°C (Liand Mathec 1994). Reprinted with permission from
Industrial and Engineering Chemistry Reseanh, Copyright 1994, American Chemical
Society
Chen and Evans (1986). Austgen et al. used the model to successfully correlate experimental
VLE data for the systems MEL4-MDEA-H20-C02 and DEA-MDEA-H20-C02. The model is
thermodynamically rigorous, but computationally complex, and has not been widely accept-
ed for design applications.
Amine Solution Vapor Pressures
Data on the total vapor pressure of amine solutions as a function of temperature and amine
concentration are necessary for the design of stripping columns and reboilers. Such data are
presented in Figure 2-56 for MEA solutions, 2-57 for DEA solutions, 2-58 for DGA solu-
tions, 2-59 for TEA solutions, 2-60 for DIPA solutions, and 2-61 for MDEA solutions. These
charts can be used to determine the boiling point of an amine solution as a function of con-
centration and pressure; however, they do not show the composition of the vapor phase.
Vapor-liquid equilibrium composition charts for MEA and DGA solutions at selected pres-
sures are given in Chapter 3. Additional data on amine solution vapor-liquid equilibrium can
usually be obtained from the manufacturers.
Heats of Reaction
Data on the heats of reaction of amines with the acid gases are necessary for the genera-
tion of individual tray and overall vessel heat balances for the absorber and stripper, the cal-
culation of the amount of steam needed in the reboiler, and the estimation of heat duties of
heat exchange equipment in the plant. As noted previously, the heats of reaction are not con-
stants for each amine and acid gas, but generally decrease as the acid gas concentration in