Page 103 - Gas Purification 5E
P. 103
Alkanolamines for Hydrogen Surfde and Carbon Dioxide Renioval b3
210 230 250 270 290 310 330 350 370
BOILING POINT. OF.
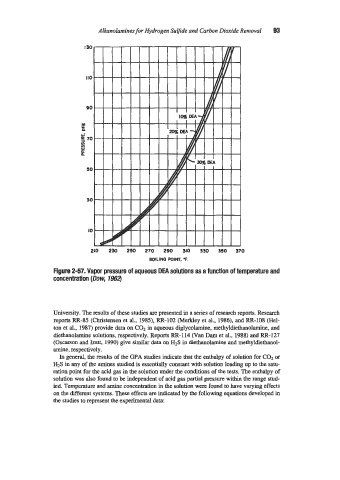
Figure 2-57. Vapor pressure of aqueous DEA solutions as a function of temperature and
concentration (Dow, 1964
University. The results of these studies are presented in a series of research reports. Research
reports RR-85 (Christensen et al., 1985), RR-102 (Merkley et al., 1986), and RR-108 (Hel-
ton et al., 1987) provide data on C02 in aqueous diglycolamine, methyldiethanolamine, and
diethanolamine solutions, respectively. Reports RR-114 (Van Dam et al., 1988) and RR-127
(Oscarson and Izatt, 1990) give similar data on H2S in diethanolamine and methyldiethanol-
amine, respectively.
In general, the results of the GPA studies indicate that the enthalpy of solution for CO1 or
H2S in any of the amines studied is essentially constant with solution loading up to the satu-
ration point for the acid gas in the solution under the conditions of the tests. The enthalpy of
solution was also found to be independent of acid gas partial pressure within the range stud-
ied. Temperature and amine concentration in the solution were found to have varying effects
on the different systems. These effects are indicated by the following equations developed in
the studies to represent the experimental data: